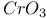|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Halides, sulfonyl reaction with alkenes 1045
Halides, sulfonyl reaction with diazomethane 1249
Halides, sulfonyl reduction and 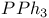 1557 1557
Halides, sulfonyl reduction with 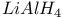 1556 1556
Halides, sulfonyl reduction with Zn and acid 1556
Halides, vinyl from acyl halides 1692
Halides, vinyl from aldehydes 1693
Halides, vinyl from alkyl halides 1692
Halides, vinyl from alkynes 1692
Halides, vinyl from allenes 1692
Halides, vinyl from halide exchange 1692
Halides, vinyl from halophosphoranes 1693
Halides, vinyl from hydrogen halides 1692
Halides, vinyl from ketones 1693
Halides, vinyl from organometallic compounds 1692
Halides, vinyl from Petasis alkenylation 1693
Halides, vinyl from scoopy reactions 1693
Halides, vinyl from Tebbe alkenylation 1693
Halides, vinyl from ylids 1693
Halides, vinyl hydrolysis of 463
Halides, vinyl reagents for preparation of 798—799
Haller-Bauer reaction 512 814
Halo acids, conversion to lactones 488
Halo alcohols, from dihalides and carbonyl compounds 1207
Halo aldehydes, from aldehydes 775
Halo alkenes, and radical cyclization 1040
Halo carbonyl compounds, formation of 1670
Halo ethers elimination reactions 1344
Halo ethers from addition of hypohalites to double bonds 1670
Halo ethers from alkenes alcohols 1056
Halo ethers from reaction of carboxylic esters with ClF 1670
Halo ketones elimination with base 1341
Halo ketones from alkenes 1048
Halo ketones from diazo ketones 522
Halo ketones from sulfones 778
Halo nitroso compounds, from alkenes 1045
Halo sulfides, and the Ramberg-Backlund reaction 1343
Halo sulfones and the Ramberg — Backlund reaction 1341
Halo-ketones, from ketones 775
Haloalkenes, reaction with organocopper compounds 423
Haloalkylation, of aromatic compounds 721
Haloamines from amines 819
Haloamines from N-haloamines 1670
Haloamines from unsaturated compounds 1670
Halocarbenes insertion reactions 789
Halocarbenes reaction with heterocycles 1088
Haloform reaction 813
Haloform reaction rate of reaction 813
Haloformic esters, from alcohols 483
Halogen dance 735
Halogenation of carboxylic acids 777
Halogenation of enamines 788
Halogenation of enol borinates 777
Halogenation of enolate anions 776
Halogenation of Grignard reagents 798
Halogenation of hydrocarbons 907—911
Halogenation of organometallic compounds 798
Halogenation photolysis 899
Halogens as neighboring groups 407
Halogens by conversion to epoxides 478
Halogens from cleavage of epoxides with hydrogen halides 1670
Halogens from epoxides 520
Halogens reaction with alkenes, reagents for 1041
Halogens reaction with conjugated compounds 1042
Halohydrins from addition of hypohalous acids to alkenes 1670
Halolactonization 1043
Halonium ions, as leaving groups 446
Hammett 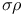 relationship 437 relationship 437
Hammett acidity function 334
Hammett equation 368
Hammett equation and electrophilic aromatic substitution 692
Hammett equation and linear free energy relationship 370
Hammett equation and radical hydrogen abstraction 903
Hammett equation and rate constants 369
Hammett equation and spectroscopy 369
Hammett equation and the selectivity relationship 692
Hammett reaction constant, and acid strength 350
Hammett relationship and 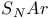 reactions 859 reactions 859
Hammett relationship and the  reaction 436 reaction 436
Hammett — Brown relationship 693
Hammond postulate 284—285
Hammond postulate and  reactions 432 reactions 432
Hammond postulate and electrophilic aromatic substitution 681
Hard acids and bases 338—342
Hard acids and bases definitions 340
Hard-soft acid-base theory 338—342
Hard-soft, acid-base principle 341
Hard-soft, acid-bases absolute hardness table 341
Hard-soft, acid-bases absolute softness 341
Hard-soft, acid-bases ambident nucleophiles 460
Hard-soft, acid-bases and ambident nucleophiles 460
Hard-soft, acid-bases and electrophilic aromatic substitution 692
Hard-soft, acid-bases HSAB principle 341
Hard-soft, acid-bases table 340
Hardness absolute, table 341
Hardness acids and bases 340
Hardness and aromaticity 52
Hardness and electrophilic aromatic substitution 692
Hardness model for aromaticity 48
Hardness quantitative definition 340
Hartree-Fock method 34
HBr, acidity in DMF 351
HBr, cleavage of ethers 465
HBr, radical addition to alkenes 991
HBr, reaction with 3-bromo-2-butanol 405 411
HBr, reaction with alkenes 991
HBr, reaction with cyclopropane 988
HBr, reaction with diazo ketones 522
HBr, reaction with ethers 520
HCl, reaction with alkenes 991
HCl, reaction with aromatic compounds and formaldehyde 721
HCl, reaction with aryl ammonium salts 729
HCl, reaction with C=N compounds 1240
HCl, reaction with N-chloroacetamides 729
HCN reaction with hydrazones 1240
HCN reaction with hydrazones addition to alkenes. 1038
HCN reaction with hydrazones reaction with aldehydes or ketones 1239
HCN reaction with hydrazones reaction with imines. 1240
HCN reaction with hydrazones reaction with nitriles 1241
HCN reaction with hydrazones reaction with oximes 1240
HCN reaction with hydrazones reaction with Schiff bases 1240
Heat of atomization 22
Heat of atomization and strain 180
Heat of atomization benzene 35
Heat of combustion 22 24
Heat of hydrogenation, benzene 35
Heat, of combustion, and elimination reactions 1315
Heck reaction 930
Heck reaction asymmetric 931
Heck reaction intramolecular 931
Heck-like coupling 937
Helical chirality 134—135
Helical shape, and chirality 134
Helicene 134
Helicenes, and optical activity 134—135
Hell-Volhard-Zelinskii reaction 777
Hemiacetals from aldehydes and alcohols 1180 1671
Hemiacetals from electrolytic oxidation of tetrahydrofuran 1671
Hemiacetals hydrolysis of 1419
Hemiaminals 1186
Hemiaminals from aldehydes or ketones and amines 1671
Hemicarcerand, and cyclobutadiene 59
Hemimercaptals from addition of thiols to aldehydes or ketones 1671
Hemimercaptals from aldehydes and thiols 1181
Hemispherands 106—107
Henkel reaction 733
Heptahelicene, resolution 153
Heptalene 134
Heptalene and aromaticity 54
Heptalene and NMR 54—55
| Heptalene derivatives 55
Herndon model for aromaticity 48
Herz reaction 704
Hess — Schaad model for aromaticity 47—48
Heteroatom Diels — Alder reactions 1075
Heteroatoms in rings, conformation 175—177
Heteroatoms, stabling effect on carbocations 223
Heterocycles acylation with aldehydes 934
Heterocycles alkylation of 871 933
Heterocycles and Birch reduction 1010
Heterocycles and electrophilic aromatic substitution 688
Heterocycles and Ziegler alkylation 871
Heterocycles aromaticity 48 51
Heterocycles by the Curtius rearrangement 1672
Heterocycles by the Diels — Alder reaction 1672
Heterocycles by the Fischer indole synthesis 1672
Heterocycles by the Hofmann — Loffler rearrangement 1672
Heterocycles by the Pschorr arylation 1671
Heterocycles by vinylcyclopropane rearrangement 1443
Heterocycles from  -unsaturated ketones 1672 -unsaturated ketones 1672
Heterocycles from  -diketones 1672 -diketones 1672
Heterocycles from  -keto esters 1672 -keto esters 1672
Heterocycles from 1,3-dipolar addition to double bonds 1672
Heterocycles from acetals 1671 1672
Heterocycles from active hydrogen compounds 1671
Heterocycles from alcohols 1671 1672
Heterocycles from aldehydes 1672
Heterocycles from alkenes 1672
Heterocycles from alkynes 1672
Heterocycles from amides 1671
Heterocycles from amines 1671 1672
Heterocycles from aminonitrenes 1672
Heterocycles from aromatic compounds 1671
Heterocycles from aromatization of heterocyclic rings 1672
Heterocycles from arylhydrazones 1672
Heterocycles from azides 1672
Heterocycles from carbenes 1672
Heterocycles from conjugated diynes 1671
Heterocycles from cycloaddition reactions 1672
Heterocycles from dienes 1672
Heterocycles from dihalides 1671
Heterocycles from diketones 1672
Heterocycles from dinitriles 1672
Heterocycles from diols 1671
Heterocycles from expansion of heterocyclic rings 1672
Heterocycles from extrusion of 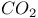 from benzoxadiazepinones 1672 from benzoxadiazepinones 1672
Heterocycles from glycols 1671
Heterocycles from haloamines 1671
Heterocycles from halohydrins 1671
Heterocycles from heteroatom Diels — Alder reactions 1672
Heterocycles from heterocycles 1671
Heterocycles from hydrazines 1672
Heterocycles from hydrogenation of heterocycles 1671
Heterocycles from intramolecular acylation 1671
Heterocycles from ketones 1672
Heterocycles from lactams 1672
Heterocycles from lactones 1672
Heterocycles from malonic esters 1671
Heterocycles from N-tosyl malonic esters 1671
Heterocycles from nitrenes 1671
Heterocycles from rearrangement of N-haloamines 1672
Heterocycles from ring expansion of ammonium salts 1672
Heterocycles from ring expansion of aryl azides 1413
Heterocycles from ring expansion of N-acylaziridines 1672
Heterocycles from sulfones 1672
Heterocycles from thiobenzilic acid 1672
Heterocycles from tosylmethylisocyanide 1672
Heterocycles from trimerization of aldehydes 1672
Heterocycles from unsaturated alcohols 1672
Heterocycles from ureas 1671
Heterocycles fromboranes 1671
Heterocycles nitration of 696
Heterocycles, reaction with amide anion 873
Heterocycles, reaction with carbenes 1087
Heterocycles, reaction with carboxylic acids 933
Heterocycles, reaction with hydrazide ions 873
Heterocycles, reaction with organolithium reagents 871
Heterocycles, reduction with indium metal 1011
Heterocycles, reduction with samarium iodide 1011
Heteropolytungstic acid and conversion of hydrocarbons to amides 925
Hexachlorocyclohexane 1301
Hexachlorocyclohexane and meso compounds 161
Hexacyclodecanes 183
Hexadecanol monosuccinate 1532
Hexaethylbenzene, from 3-hexyne 1090
Hexahelicene, and strain 190
Hexaisopropylbenzene 189 1089
Hexaisopropylcyclohexane, conformation 174
Hexamethylbenzene 1084
Hexamethylbicyclo[2.2.0]hexadiene 1083
Hexamethylenetetramine and oxidation of alkyl halides 1536
Hexamethylenetetramine and the Duff reaction 717
Hexamethylenetetramine reaction with alkyl halides 501
Hexamethylphosphoramide see “HMPA”
Hexamethylprismane 1083
Hexaphenylethane 241
Hexaydroazepin-2-ones, conformation 178
HF, reaction with acyl halides 524
HF, reaction with anhydrides 524
HI and reduction of alkyl halides 518
HI, cleavage of ethers 465
HI, gas phase addition to dienes 992
HI, reaction with alkenes 991
HI, reaction with ethers 519
High pressure and epoxide hydrolysis 468
High pressure and formation of oximes 1194
High pressure and reaction of epoxides with ammonia 504
High pressure and steric hindrance 458
High pressure and the Claisen rearrangement 457
High pressure and the Cope rearrangement 457
High pressure and the Diels — Alder reaction 457 1066
High pressure and the Heck reaction 931
High pressure and the Knoevenagel condensation 457
High pressure and the Menshutkin reaction 458
High pressure and volume of activation 457
High pressure reactions 457—458
Higher order cuprates see “Organocuprates”
Highest occupied molecular orbital see “HOMO”
Hindrance, steric see “Steric hindrance”
Hinsberg test, and amines 576
HMPA 236
HMPA and 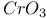 1515 1515
HMPA and aggregation of bases 349
HMPA and alkylation of amines 503
HMPA and coupling of all ylie halides 542
HMPA and dehydration of alcohols 1327
HMPA and dehydrohalogenation 1337
HMPA and formation of aniline derivatives 864
HMPA and halide in substitution with tosylates 518
HMPA and hydrogen bonding 100
HMPA and hydrolysis of sulfonamides 576
HMPA and reaction of aryl halide and alkoxides 862
HMPA as a solvent 450
HMPA formation of thiophenols 863
HMPA solvent for alkylation reactions 488
HOBr see “Hypobromous acid”
HOC1 see “Hypochlorous acid”
Hoesch reaction 723
Hofmann degradation 1331
Hofmann elimination 1331
Hofmann rearrangement 1380 1384
Hofmann — Loffler reaction 909 1462
Hofmann — Loffler — Freytag reaction 1462
Hofmann — Martius reaction 729
Hofmann’s rule 1315 1332
Hofmann’s rule and elimination of alkyl halides 1337
Hofmann’s rule and pyrolytic eliminations 1325
Hofmann’s rule and substituent effects on elimination 1320
HOI see “Hypoiodous acid”
HOMO alkenes 1070
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



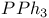
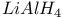
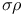 relationship
relationship 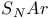 reactions
reactions  reaction
reaction  -unsaturated ketones
-unsaturated ketones  -diketones
-diketones 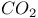 from benzoxadiazepinones
from benzoxadiazepinones