|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Mechanisms, SET transmetallation 803
Mechanisms, SET types 274—275
Mechanisms, SET von Braun reaction 523
Mechanisms, SET von Richter rearrangement 877
Mechanisms, SET Wacker process 1538
Mechanisms, SET Wallach rearrangement 1465
Mechanisms, SET Willgerodt reaction 1567
Mechanisms, SET Wittig reaction 1234
Mechanisms, SET Wittig rearrangement 1421
Mechanisms, SET Wohl — Ziegler bromination 912
Mechanisms, SET Wolff rearrangement 1406
Mechanisms, SET Wolff — Kishner reduction 1548—1549
Mechanisms, SET [2+2]-cycloadditions 1078
Mechanisms, SET [3+2]-cycloadditions with allylic anions 1076
Medium effects, on elimination reactions 1321
Medium rings, and strain 185
Medium, and acid-base strength 349—351
Medium, reaction see “Reaction medium”
Meerwein arylation 929—930
Meerwein reaction 928
Meerwein — Ponndorf — Verley reduction 1199
Meerwein — Ponndorf — Verley reduction and Oppenauer oxidation 1516
Meinwald rearrangement 1398
Meisenheimer rearrangement 1420
Meisenheimer salts 851
Meisenheimer — Jackson salts 851
Meldram’s acid,  347 347
MEM, protecting groups 478
Memory effects 1386
Menshutkin reaction 393 499
Menshutkin reaction and high pressure 458
Menthyl chloride, E2elimination 1302
Mercaptans see “Thiols”
Merck Index 1618
Mercuric acetate, reaction with bicyclic amines 1512
Mercuric chloride, reaction with boronic ester salts 1424
Mercuric oxide, and hydration of alkynes 995
Mercuric sulfate, and hydration of alkenes 995
Mercury fulminate, reaction with aromatic compounds 723
Mesitoic acid, mechanism of hydrolysis 472
Mesitylene, reaction with ethyl fluoride and 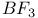 678 678
Mesitylene, reaction with peroxyacids 724
Meso compounds 145 (also see “Diastereomers”)
Meso compounds and cis/trans isomers 161
Meso compounds and E2elimination 1301
Meso compounds and erythro/threo nomenclature 147
Mesoionic compounds, and aromaticity 69—70
Mesomeric effect 42 (also see “Resonance”)
Mesylate, leaving group 446
Mesylates, di-, elimination reactions 1340
Mesylates, reaction with carboxylic acid salts 489
meta-substitution 681
Metal carbenes see “Carbenes”
Metal carbenes, and alkene metathesis 1458
Metal complexes, of cyclobutadiene 60
Metal halides, and transmetallation 803
Metal hydride addition-elimination mechanism 772
Metal hydrides, reaction with alkenes 1016
Metallation, of alkenes 791
Metallocenes 53—54
Metallocenes and chirality 135
Metallocenes and transmetallation 803
Metallocenes electron donor-acceptor complexes 103
Metallocenes from sodium cyclopentadienylid and metal halides 1681
Metals and elimination of dihalides 1343
Metals and reduction of alkenes 1008
Metals dissolving metal reduction, alkynes 1008
Metals dissolving metal reduction, aromatic compounds 1009
Metals reaction with acidic hydrocarbons 793
Metals reaction with alkyl halides 805
Metals reaction with aryl halides 805
Metals reaction with organometallics 802
Metathesis alkene 1457—1459
Metathesis alkene, and ring expansion 1458
Metathesis alkene, substituent effects 1457
Metathesis alkenes, intramolecular 1458
Metathesis with alkynes 1458
Methane andPES 10—12
Methane bond angles 8 21
Methane bond energy 23
Methane heat of combustion 22
Methane hybridization 8
Methane N-methanesulnnyl- -toluidine, and the Knoevenagel reaction 1226 -toluidine, and the Knoevenagel reaction 1226
Methanesulfonate, leaving group 446
Methanesulfonyl chloride, reaction with aniline, sulfene intermediate 575
Methanonium ion 218 770
Methoxymethyl cation 223
Methoxyvinyllithium, in the Knoevenagel reaction 1227
Methyl benzoyl formate 149
Methyl carbanion 229 232
Methyl carbinols see “Alcohols methyl”
Methyl esters, from carboxylic acids and diazomethane 490
Methyl groups, oxidation of 1533
Methyl hydrocarbons, from ester 1552
Methyl ketones see “Ketones methyl”
Methyl radical 238 244
Methyl sulfate, reaction with alkoxides 478
Methyl transfer 287
Methyl vinyl ketone see “Ketone methyl
Methyl(trifluoromethyl)dioxirane 1533
Methylamine, base strength 349
Methylation of alcohols,with diazomethane 479
Methylation of amines with diazomethane 504
Methylation of aryls 937
Methylation of carboxylic acids 489
Methylation of ketones, by the Simmons — Smith reaction 1089
Methylation of qninoline 872
Methylcerium dichloride 1215
Methylcyclohexane, and conformations 173
Methylene carbene 248
Methylene carbene from diazomethane 789
Methylene insertion 788
Methylene, from diazomethane 1087
Methylenecyclopropanes 1077
Methyllithium 234
Methyllithium reaction with enol acetates 554
Methyllithium reaction with enol borinates 554
Methyllithium reaction with silyl enol ethers 554
Methylpyridinium salts, reaction with phosphines 501
Methylsulflnyl carbanion and reaction of alcohols with 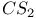 1184 1184
Methylsulflnyl carbanion reaction with aromatic nitro compounds 872
Methylsulflnyl carbanion reaction with ester 572
Methylsulflnyl carbanion reaction with esters 572
Mexican bentonite clay, and formation of nitriles 1195
Meyer — Schuster rearrangement 423
Meyers synthesis 558
Micellar catalysis, and alkylation reactions 477
Michael addition 976 (also see “Addition conjugate” “Addition Michael”)
Michael addition and chiral additives 1023
Michael addition and enolate anions 1023
Michael reaction 976 1022—1024 1221
Michael reaction and Mannich bases 1023
Michael reaction and the Stork enamine reaction 787
Michaelis — Arbuzov rearrangement 1289 (ref. 750)
Microscopic reversibility 285—286
Microscopic reversibility and E1cB elimination 1310
Microwave spectroscopy, and bond distance 18
Microwaves and acetal hydrolysis 467
Microwaves and alkylation of amines 501
Microwaves and chemical reactivity 457
Microwaves and dehydration of oximes 1348
Microwaves and ester hydrolysis 470
Microwaves and esterification 484
Microwaves and formation of enamines 1186
Microwaves and formation of Mannich bases 1191
Microwaves and formation of nitriles 1195
Microwaves and hydrolysis of C=N compounds 1177
Microwaves and isocyanate-imine cycloaddition 1251
Microwaves and nucleophilic substitution 457
Microwaves and oxidation of alcohols 1515
| Microwaves and preparation of alkyl halides 518
Microwaves and reductive alkylation of amine 1188
Microwaves and the Baylis — Hillman reaction 1212
Microwaves and the Beckmann rearrangement 1415
Microwaves and the Diels — Alder reaction 1065
Microwaves and the Heck reaction 931
Microwaves and the Knoevenagel reaction 1226
Microwaves and the Krohnke reaction 1536
Microwaves and the Meerwein — Ponndorf — Verley reduction 1266 (ref. 294)
Microwaves on 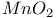 1572 (ref. 53) 1572 (ref. 53)
Microwaves with 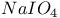 on silica gel 1589 (ref. 449) on silica gel 1589 (ref. 449)
Migration in cleavage of aromatic compounds 731
Migration of an ipso substituent 687
Migration of phenyl groups 732
Migration of vinyl groups in radicals 1391
Migration with borane an hydroperoxide anion 797
Migratory aptitudes and electronic effects 1386
Migratory aptitudes for aryl groups and carbocations 1386
Migratory aptitudes in nucleophilic rearrangements 1384—1386
Migratory aptitudes in radical rearrangements 1389
Migratory aptitudes in the Baeyer — Villiger rearrangement 1417
Migratory aptitudes in the Beckmann rearrangement 1415
Migratory aptitudes in the pinacol rearrangement 1385
Migratory aptitudes in the Wittig rearrangement 1421
Mills reaction 818
Mills — Nixon effect, and electrophilic aromatic substitution 690
MINDO/3method 34
Mirror plane 127—128
Mislow — Evans rearrangement 1455
Mitsunobu esterification reaction 486
Mitsunobu esterification, catalysts for 486
Mitsunobu reaction, and amine formation 502
Mitsunobu reaction, formation of amides 513
Mixed anhydrides 508
Mixed cuprates see “Organocuprates”
MM2 calculations 179
MM3 calculations 179
MMP2 calculations 179
MNDO method 34
MO 4 32—35
MO ab initio, and bromonium ions 973
MO and ion pairs 399—400
MO and regioselectivity in the Diels — Alder reaction 1064
MO and small rings 181
MO and the frontier orbital method 1068—1070
MO and the Mobius — Huckel method 1070
MO basis sets 1070
MO Hueckel 34
MO of benzene 33—34
MO of benzene, and electrophilic aromatic substitution 683
Mobius strip 136
Mobius strip and Diels — Alder reactions 1071
Mobius strip mechanism, and catenaries 113
Mobius systems 1071
Mobius — Huckel method 1070 1431
Mobius — Huckel method and electrocyclic rearrangements 1429
Mobius — Huckel method and [1,3]- and [1,5]-H sigmatropic rearrangements 1439
Molecular knots 114
Molecular knots and chirality 136
Molecular knots and potential energy 179
Molecular knots formal steric enthalpy 190
Molecular knots Molecular mechanics 178—180
Molecular mechanism, and conformations 167
Molecular orbitals see “MO”
Molecular recognition 108
Molecular shuttles, and rotaxanes 114
Molecularlity and mechanism 291
Molecularlity definition 291
Molecules, electronic structures 12—13
Molybdenum peroxide reagent, and allylic oxidation 915
MOM, protecting group 478
Mono esters see “Carboxylic acids mono
Monocyclic compounds see “Cyclic compounds”
Montmorillonite K10 clay 457
Montmorillonite K10 clay and acetal hydrolysis 467
Montmorillonite K10 clay and Friedel — Crafts reactions 708
Montmorillonite K10 clay and preparation of alkyl halides 518
Montmorillonite K10 clay and the Beckmann rearrangement 1415
Montmorillonite K10 clay and the Heck reaction 931
Montmorillonite K10 clay and the Prins reaction 1242
Montmorillonite K10 clay and the Willgerodt reaction 1567
Montmorillonite K10 clay and transesterification 486
Montmorillonite K10 clay to form  -amino esters 1191 -amino esters 1191
Morphine, and resolution 151
Mosher’s acid 142—143
MPTA see “Mosher’s acid”
Mukaiyama aldol reaction 1223
Mukaiyama reagent 1223
Mukaiyama reagent and dehydration of carboxylic acids 1327
Mukaiyama reagent and lactonization 485
Multiple bonds see “bonding multiple”
N,N,N’,N’-Tetramethyle1hylenediaimne 236
N,N-Dichloroamines, rearrangement of 1410
N,N-Diethylcarbamoyl chloride, reaction with aromatic compounds 718
N,N-Dimethylacetamide 295
n-Benzoylnorephedrine 366
N-Bromosuccinimide see “NBS
N-Chloro-2-azabicyclo[2.2.2]octane 1416
N-Chloroacetamides, rearrangement of 728—729
N-Chloroamines see “Amines N-chloro”
N-Chloromethyl lactams, reaction with amines 500
N-Chlorosuccinimide see “NCS”
N-Methyl-N-benzylthiomesitylide 158
N-nitroamines, rearrangement of 727
N-Nitrosoamines from amines 818
N-Nitrosoamines rearrangement of 728
N-romosuccinimide see “NBS”
Nafion-H and Friedel — Crafts reactions 708
Nafion-H and hydration of alkynes 995
Nametkin rearrangement 1394
Naphthalene and aromaticity 49
Naphthalene and Friedel — Crafts 709
Naphthalene bond order 49
Naphthalene derivatives, and electrophffic aromatic substitution 689—690
Naphthalene oxidation of 1527
Naphthalene resonance energy 49
Naphthalene sulfonation of 682
Naphthalenesulfonic acid 682
Naphtho(b)cyclobutene 1355
Naphthol, reaction with ammonia 865
Naphthylamines, by the Bucherer reaction 865
Naphthylamines, from naphthols 865
Nazarov cyclization 1021
NBS and bromine 913
NBS and bromolactamization 1043
NBS and radical bromination 911
NBS and Wohl — Ziegler bromination 911
NBS bromination of aldehydes 914
NBS halogenation of enol borinates 777
NBS reaction with ammonia and aldehydes 925
NBS reaction with carboxylic acids 778
NBS reaction with sulfones 778
NCS and TEMPO, oxidation of alcohols 1515
NCS reaction with amines 500
NCS reaction with carboxylic acids 778
NCS reaction with sulfones 778
Neber rearrangement 288 1410
Nef reaction 1178 1225
Negative enhancement, and radicals, in NMR 240
Neighboring group effects and electrophilic aromatic substitution 412
Neighboring group effects and radical substitution 899
Neighboring group effects and solvolysis 410
Neighboring group effects cyclobutylmethyl 417
Neighboring group effects cyclopropyl rings 410
Neighboring group effects cyclopropylmethyl 418
Neighboring group effects hydrogen 419
Neighboring group effects in rearrangements 1381
Neighboring group effects in the Prins reaction 1241
Neighboring group effects migration of amino groups 1409
Neighboring group effects norbornyl compound 414
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте




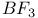
 -toluidine, and the Knoevenagel reaction
-toluidine, and the Knoevenagel reaction 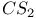
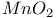
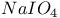 on silica gel
on silica gel