|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Imine salts hydrolysis of 1217
Imine-enamine tautomerism see “Tautomerism”
Imines and oxa-di- -methane rearrangements 1461 -methane rearrangements 1461
Imines and the Ugi reaction 1252
Imines azomethine, as 1,3-dipoles 1060
Imines by the heteroatom Diels alder reaction 1676
Imines by the Stieglitz rearrangement 1417 1676
Imines cyclic, from azides 1413
Imines cyclic, from halonitriles 1217
Imines cyclic, from organometallics 1217
Imines dimerization, reagents for 1561
Imines from active hydrogen compounds 1676
Imines from aldehydes 1185—1187 1676
Imines from alkyl azides 1412
Imines from alkynes 1676
Imines from amide dehydration 1327
Imines from amides 1327 1677
Imines from amines 1185—1187 1417 1519 1536 1676
Imines from ammonia 1676
Imines from azides 1389 1676
Imines from carboxylic acids 532
Imines from enamines 1676
Imines from Grignard reagents 1676
Imines from isocyanates 1677
Imines from isonitriles 1676
Imines from ketones 1185—1187 1676
Imines from metalated imines 1676
Imines from N-haloamines 1417
Imines from nitriles 1676
Imines from nitrilium salts 1676
Imines from nitroso compounds 1676
Imines from organometallic compounds 1676
Imines from oxime s 1676
Imines from oxime sulfonates 1676
Imines from phosphoranes 1677
Imines from rearrangement of functionalized amines 1676
Imines from ylids 1676
Imines hydrolysis of 532 1177
Imines in the aldol reaction 1222
Imines intermediates in the Schmidt reaction 1414
Imines isolation of 1217
Imines metalated 1252
Imines nitrile, as 1,3-dipoles 1060
Imines polymerization of 1186
Imines preparation using drying agents 1186
Imines preparation via azeotropic distillation 1186
Imines reaction with 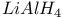 1203 1203
Imines reaction with amines 1001
Imines reaction with base and an aldehydes 1222
Imines reaction with chlorosulfonyl isocyanate 1251
Imines reaction with Grignard reagents 1216
Imines reaction with HCN 1240
Imines reaction with ketenes 1250
Imines reaction with phosphorus ylids 1237
Imines reduction, reagents for 1203
Imines tantomerism with enamines 77
Iminium ions and nucleophilic addition 1178
Iminium ions and the Mannich reaction 1190
Iminium salts and the Stephen reduction 1204
Iminium salts conversion to nitriles 722—723
Iminium salts from alcohols 1183
Iminium salts from aldehydes 1187
Iminium salts from amine perchlorates 1187
Iminium salts from ketones 1187
Iminium salts from nitriles 1183
Iminium salts from oxazines 559
Iminium salts hydrolysis of 787 1183
Iminium salts in the Gatterman reaction 715
Iminium salts reaction with 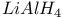 1203 1203
Iminium salts reaction with Grignard reagents 1216
Iminonitriles, from nitriles 1241
In-in isomers 163
In-out isomers 163
Inclusion compounds 109—111
Inclusion compounds and carcerands 111
Inclusion compounds and cyclodextrins 111—113
Inclusion compounds and hydrazine 111
Inclusion compounds and hydroquinone 110—111
Inclusion compounds and molecular knots 114
Inclusion compounds and rotaxanes 114
Inclusion compounds cage compounds 109
Inclusion compounds clathrates 109
Inclusion compounds definition 109
Indacene 68
Indene,  52 52
Indium trichloride, and the Diels-Alder reaction 1066
Indium, reduction of heterocycles 1011
Indole, and electrophilic aromatic substitution 689
Indoles by Fischer indole synthesis 1452—1453
Indoles by the Fisher indole synthesis 1452
Indoles from arylhydrazones 1452
Indoles reaction with carbenes 1087
Inductive effects 16—18
Inductive effects and hybridization 18
Inductive effects influence on  reactions 436 reactions 436
Inductive effects –I and+I 17—18
Infrared spectroscopy and carbenes 249 1086
Infrared spectroscopy cyclohexyne 187
Infrared, and hydrogen bonding 100
Ing — Manske procedure 513
Inhibitors and radicals 896
Inhibitors radical, AIBN 911
Inhibitors radical, peroxides 911
Inorganic esters, hydrolysis of 464
Insertion reactions intramolecular 789
Insertion reactions intramolecular, of carbocations 787
Insertion reactions of carbenes 251 788—791
Insertion reactions of carbenoids 791
Insertion reactions of methylene 788
Insertion reactions of nitrenes 253—254 782
Interconversion 1,3-diene-cycIobutene 1430
Interfacial mechanism, phase transfer catalysis 455
Intermediate mechanisms, for  reactions 401 reactions 401
Intermediates and CIDNP 288
Intermediates and energy diagrams 281—282
Intermediates and mechanism 281—282
Intermediates and the  reaction 393 reaction 393
Intermediates and the Hammond postulate 285
Intermediates aryl radical rearrangement 1390
Intermediates azirine, in the Neber rearrangement 1410
Intermediates bisperoxide, and ozonolysis 1523
Intermediates cyclopropane, in the Claisen rearrangement 1451
Intermediates detection of 288—289
Intermediates diazo ketones, in the Schmidt reaction 1414
Intermediates dihydrophenanthrene, in photocyclization of stilbene 1436
Intermediates episulfones, in the Ramberg-Backlund reaction 1342
Intermediates esters, in oxidation-reduction 1508
Intermediates in amide hydrolysis 365
Intermediates in the Reformatsky reaction 1212
Intermediates isolation of  intermediates 851 intermediates 851
Intermediates ketenimine, in the Schmidt reaction 1414
Intermediates ketocarbene, in Wolff rearrangement 1407
Intermediates nitrenes, in the Curtius rearrangement 1412
Intermediates nitrenes, in the Hofmann rearrangement 1411
Intermediates nitrenes, in the Lossen rearrangement 1413
Intermediates ozonolysis, from photooxidation of diazo compounds 1524
Intermediates protonated cyclopropane 1382
Intermediates sulfene 575 1338
Intermediates Wheland 676
Internal aldol see “Aldol intramolecular”
Internal conversion 313
Internal return, and ion pairs 398
Internal strain see “Strain I”
International Union of Pure and Applied Chemistry see “IUPAC”
Intersystem crossing 315
Intimate ion pairs 398
Intramolecular, addition of radicals 985
Intrinsic barrier 286
Intrinsic free energy 286
Inverse electron demand, in Diels-Alder reaction 1067
| Inverse isotope effects 298
Inversion of carbanions 233
Inversion Walden 290
Iodide ion and elimination of dihalides 1343
Iodide ion radiolabeled, in  reactions 392 reactions 392
Iodide ion reaction with ditosylamines 522
Iodides see “Halides”
Iodides, alkyl by the Katritzky pyrylium-pyridinium method 522
Iodides, alkyl from amines 522
Iodides, alkyl from esters 521
Iodides, alkyl from ethers and III 519
Iodides, vinyl, from alkynes and boranes 798
Iodination of aryl diazonium salts 875
Iodination of ketones and aldehydes 776
Iodine and electrophilic aromatic substitution 706
Iodine and radical substitution 908
Iodine and the Simonini reaction 943
Iodine azide, reaction with alkenes 1046
Iodine hypervalent, and ester formation 488
Iodine hypervalent, and oxidation of alcohols 1516
Iodine hypervalent, lactone formation 488
Iodine reaction with alkenes and Chloramine-T 1058
Iodine reaction with alkynes 1042
Iodine reaction with boranes 798
Iodine reaction with methyl ketones 813
Iodine reaction with vinyl boranes and NaOH 1424
Iodo azides conversion to aziridines 1046
Iodo azides from alkenes 1046
Iodoethane reaction with amines and 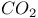 820 820
Iodoform, by the haloform reaction 813
Iodolactamization 1043
Iodosylbenzene, oxidation of alkenes 915
Iodotrimethylsilane, and homologation of aldehydes and ketones 1407
Ion exchange resins, and the aldol reaction 1220
Ion pairs and allylic rearrangement 421—422
Ion pairs and E1 elimination 1308
Ion pairs and E2elimination 1306
Ion pairs and El cB elimination 1312
Ion pairs and internal return 398
Ion pairs and MO calculations 399
Ion pairs and racemization 399
Ion pairs and radiolabeled oxygen 398
Ion pairs and solvolysis 398
Ion pairs and stereochemistry 398
Ion pairs and the  mechanism 768 mechanism 768
Ion pairs and the  reaction 397—400 reaction 397—400
Ion pairs and [2,3]-sigmatropic rearrangement 1454
Ion pairs in the  reaction 397 reaction 397
Ion pairs intimate/contact/tight 398
Ion pairs loose/solvent-separated 398
Ion pairs scrambling of radiolabeled oxygen 399
Ion pairs special salt effect 399
Ion-pair mechanism 400
Ion-pair mechanism and the Sommelet Hauser rearrangement 878
ionic bond see “Bonding ionic”
Ionic strength, and reactivity 451
Ionization and stain 366
Ionization and the  mechanism 763 mechanism 763
Ionization of tosylates, solvent effects 451
Ionization potential and electronegativity 15
Ionization potential and PES 10—11
Ionization values, thermodynamic, for acids 350
Ionizing power and charge transfer UV peak 453
Ionizing power of solvents 452
Ionizing power of solvents, table 453
Ions aminium radical 701
Ions arenium 1402
Ions bromonium 971
Ions carbenium 219
Ions carbocations 218—227
Ions carbonium 218
Ions diazonium 448
Ions nonclassical 407—420
Ions radical 247
Ions Wheland intermediates 676
Ipso attack and electrophilic aromatic substitution 686
Ipso attack migration 687
Ipso position, and electrophilic aromatic substitution 686
Ipso substitution, and aryl radicals 905
IR see “Infrared”
Ireland — CIaisen rearrangement 1452
Iron pentacarbonyl reaction with Na-Hg 562
Iron pentacarbonyl reaction with RMgX and RLi 801
Isoborneol 1394
Isobutane, bond energy 23
Isobutyl radical 1390
Isobutylamine, labeled, and carbocations 1383
Isochronous, definition 165
Isocyanates by the Curtius rearrangement 1412 1677
Isocyanates by the Hofmann rearrangement 1411 1677
Isocyanates by the Lossen rearrangement 1413 1677
Isocyanates by the Schmidt reaction 1413
Isocyanates by the Schmidt rearrangement 1677
Isocyanates from acyl azides 1412
Isocyanates from alkyl halides 516
Isocyanates from amides 515 1411 1677
Isocyanates from amines 507 1677
Isocyanates from carboxylate salts 1247
Isocyanates from carboxylic acids 1677
Isocyanates from cyanate ion 1677
Isocyanates from hydrazoic acid 1677
Isocyanates from nitrile oxides 1465
Isocyanates from rearrangement of acyl azides 1677
Isocyanates from rearrangement of hydroxamic acids 1677
Isocyanates hydrolysis of 1178 1411 1413
Isocyanates reaction with alcohols 1182—1183
Isocyanates reaction with amines 1191
Isocyanates reaction with ammonia 1191
Isocyanates reaction with aromatic compounds 719
Isocyanates reaction with dimethyl dioxirane 1540
Isocyanates reaction with enamines 1250
Isocyanates reaction with Grignard reagents 1217
Isocyanates reaction with phosphine oxides 1246
Isocyanates reaction with phosphorous ylids 1237
Isocyanates reaction with vinyl tin compounds 1218
Isocyanates reduction of 1557
Isocyanates reduction with 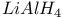 1555 1555
Isocyanic acid reaction with amines 1191
Isocyanic acid reaction with carbamates 1183
Isocyanides see “Isonitriles”
Isoinversion 765
Isomer distribution, in electrophilic aromatic substitution 691
Isomerization cis/trans, in radical addition to alkenes 979
Isomerization E/Z 991
Isomerization fluxional 160
Isomerization in carbenes 249
Isomerization in dienes 991
Isomerization of 2-azirines to 1-azirines 1058
Isomerization of alkenes see “Alkenes double
Isomerization of azo compounds 700
Isomerization of boranes 773
Isomerization of double bonds in alkenes 990
Isomerization of epoxides 481
Isomerization photochemical induced 319—320
Isomers alkenes 157—160
Isomers and rotaxanes 113
Isomers atropisomers 132
Isomers cis/trans 156—163
Isomers cis/trans, and E2 elimination 1304
Isomers cis/trans, and electrophilic addition to alkenes 971—972
Isomers cis/trans, and elimination of ammonium salts 1333
Isomers cis/trans, and elimination reactions 1317—1318
Isomers cis/trans, and ozonolysis 1524
Isomers cis/trans, and the Shapiro reaction 1335
Isomers conformational 167
Isomers conformational, cryptands 106
Isomers out-in 163
Isomers topological stereocatenanes 114
Isomers valence bond 160 1083
Isonitriles and hydrogen bonding 101
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -methane rearrangements
-methane rearrangements 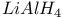
 reactions
reactions  reactions
reactions  intermediates
intermediates 
 mechanism
mechanism