|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Carboxylic acids from the haloform reaction 1661
Carboxylic acids from unsaturated carboxylic acids 1661
Carboxylic acids fromalkenes 1035—1036 1661
Carboxylic acids fromboranes 1424 1661
Carboxylic acids fromTebbe alkenylation 1661
Carboxylic acids, amino see “Amino acids”
Carboxylic acids, gas phase acidity 350
Carboxylic acids, methylation with 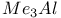 1214 1214
Carboxylic acids, monoesters 483 487
Carboxylic acids, oxidation with peroxides 1542
Carboxylic acids, oxidative decarboxylation 1528
Carboxylic acids, pyrolysis with  573 573
Carboxylic acids, reaction with  and alcohols 1184 and alcohols 1184
Carboxylic acids, reaction with acyl halides 523
Carboxylic acids, reaction with alcohols 484—486
Carboxylic acids, reaction with alkenes 997
Carboxylic acids, reaction with alkynes 998
Carboxylic acids, reaction with ammonia 508
Carboxylic acids, reaction with boranes 1005
Carboxylic acids, reaction with bromine 777
Carboxylic acids, reaction with carbonyls and isonitriles 1251
Carboxylic acids, reaction with copper 732
Carboxylic acids, reaction with diazo compound 490
Carboxylic acids, reaction with diazomethane 490
Carboxylic acids, reaction with dimethylformamide acetals 489
Carboxylic acids, reaction with enol esters 491
Carboxylic acids, reaction with esters 491
Carboxylic acids, reaction with ethers 486
Carboxylic acids, reaction with heterocycles 933
Carboxylic acids, reaction with hydrazoic acid 1413
Carboxylic acids, reaction with LDA 555
Carboxylic acids, reaction with lead tetraacetate and cupric acetate 1528
Carboxylic acids, reaction with NBS or NCS 778
Carboxylic acids, reaction with organolithium reagents 568
Carboxylic acids, reaction with oxalyl chloride 523
Carboxylic acids, reaction with pyridine 933
Carboxylic acids, reaction with quinolines 933
Carboxylic acids, reaction with thionyl chloride 523
Carboxylic acids, reaction with vinyl boranes 1005
Carboxylic acids, reduction with 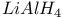 1549 1549
Carboxylic acids, reduction with borane 1549
Carboxylic acids, reduction with Li and amines 532
Carboxylic acids, selective reduction 533
Carboxylic acids, table of  343 343
Carboxylic acids, thermolysis of 1327
Carboxylic esters see “Esters”
Carbyne 249
Carcerand and cyclobutadiene 59
Carcerands and inclusion compounds 111
Caro’sacid 1539
Carvone, photochemistry of 1082
Carvonecamphor 1082
CAS registry numbers 1632
Catalysis acid — base 336—338
Catalysis and mechanism 289
Catalysis and the Bronsted relation 371
Catalysis and the Marcus equation 337
Catalysis base catalyzed reactions 337
Catalysis base, and the Knoevenagel reaction 1225
Catalysis basic, of 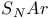 reactions 852 reactions 852
Catalysis Bronsted catalysis equation 337
Catalysis chiral, quinidine 1249
Catalysis for alkene metathesis 1457
Catalysis for amine alkylation 501
Catalysis for dihydroxylation of alkenes 1049
Catalysis for Friedel — Crafts reaction 708
Catalysis for hydrogenation 1003
Catalysis for preparation of aryl halides 502
Catalysis for the Cope rearrangement 1446
Catalysis for the ene reaction 1022
Catalysis for the Mukaiyama aldol reaction 1223
Catalysis general 336—337
Catalysis heterogeneous 1003
Catalysis hydrogenation, and aromatization 1510
Catalysis hydrogenation, for aldehydes or ketones 1198
Catalysis in the Diels — Alder reaction 1065
Catalysis in the tetrahedral mechanism 427
Catalysis Lindlar 1004
Catalysis nucleophilic, pyridine 576
Catalysis of keto — enol tautomerism 774
Catalysis of [2+2]-cycloadditions 1083
Catalysis phase transfer 454—456; see “Phase transfer catalysis”
Catalysis Rosenmund 1005
Catalysis specific 336—337
Catalysis specific acid 337
Catalysis, optical active, and the Prins reaction 1242
Catalysts and  -bond rearrangements 1459 -bond rearrangements 1459
Catalytic hydrogenation see “Hydrogenation”
Catechol 1632
Catechol, oxidation to qumones 1518
Catecholborane 798 1015
Catechols, oxidative cleavage 1527
Catenanes 113—114
Catenanes and chirality 136
Catenanes and topological stereoi somers 114
Catenanes by acyloin condensation 1563
Catenanes definition 113
Catenanes from acyloin condensation or other methods 1663
Catenanes Mobius strip mechanism 113
Cation exchange resins, and acetal hydrolysis 467
Cation radicals 247
Cation radicals and the Diels — Alder reaction 1065
Cation radicals Cationic Diels — Alder reactions 1065
Cationotropic rearrangements 1377
Cations see “Carbocations”
Cations acyl 223
Cations radical, aminium 909
Cavitation, and ultrasound 456
Cbz see “Carbobenzoxy”
Cellosolve 481
Center of symmetry 127—128
Centrohexacyclines 213
Ceric ammonium nitrate, reaction with epoxides and NH4SCN 1248
Cerium trichloride, reaction with organolithium reagents 1215
Chain reactions and substitution 403
Chain reactions radical 895
Chain reactions radical addition to alkenes 977
Chain reactions radical and pyrolytic elimination 1324
Chair conformation 172
Chair conformation and the Cope rearrangement 1446
Chair — to-chair interconversion 173
Channel — type complexes, and cyclodextrins 113
Chapman rearrangement 1464
Charge distribution, in benzenonium ion 685
Charge transfer UV peak, and ionizing power 453
Charge — transfer spectrum, EDA complexes 102
Chelation, in reactions of ketones with magnesium methyl carbonates 1229
Cheletropic reactions 1342
Chemical Abstracts 1611
Chemical processes, photochemical 317—321
Chemical reactivity and microwave irradiation 457
Chemical reactivity and ultrasound 456—458
Chemical shifts, table, for carbocations 225
Chemically induced dynamic nuclear polarization see “CIDNP”
Chemoselectivity definition 525
Chemoselectivity epoxide reduction 529
Chemoselectivity hydride reduction of aldehydes and ketones 1198
Chemoselectivity in Grignard reactions 1206
Chichibabin reaction 873
Chiral additives in the Michael reaction 1023
Chiral additives in the Reformatsky reaction 1212
Chiral additives reaction of amines and diazoalkanes 1217
Chiral additives sparteine, with organolithium reagents 1215
Chiral auxiliaries in addition of organolithium reagents to imines 1216
Chiral auxiliaries in the Reformatsky reaction 1212
Chiral auxiliary 149
Chiral axes 131—134
Chiral carbenoids 249
Chiral catalysts and synthesis 150
Chiral catalysts quinidine 1249
| Chiral centers, from reduction of ketones 1200
Chiral pool 147
Chiral recognition 152
Chiral solvents and NMR 156
Chiral solvents and synthesis 150
Chiral templates, and conjugate additions 1029
Chirality 125—139 (also see “Absolute configuration”)
Chirality and adamantanes 131
Chirality and allenes 133—134
Chirality and amines 129—130
Chirality and asymmetric carbons 128
Chirality and atropisomers 132
Chirality and biphenyls 131—132
Chirality and calixaromatic compounds 136
Chirality and catenaries 136
Chirality and crown ethers 136
Chirality and helical shape 134
Chirality and helicenes 134—135
Chirality and metallocenes 135
Chirality and molecular knots 136
Chirality and paracyclophanes 135—136
Chirality and rotaxanes 136
Chirality and spiranes 134
Chirality and stereogenic atoms 128
Chirality and stereoisomers 125—139
Chirality and tervalent atoms 129
Chirality and TrSger’s base 130
Chirality categories of optically active compounds 128—136
Chirality definition 125
Chirality helical 134—135
Chirality levo and dextro isomers 125
Chirality pseudoasymmetric carbons 145
Chirality transfer, and [2,3]-sigmatropic rearrangement 1454
Chiraphos 1029
Chloramine, reaction with boranes 800
Chloramine-T, reaction with alkenes 1056
Chloramine-T, reaction with alkenes and iodine 1058
Chloranil 1511
Chlori nation allylic 914
Chlori nation of anisole, in cyclodextrin 686
Chlori nation of aromatic rings, and arenium ions 679
Chlori nation of hydrocarbons 907—911
Chlori nation of ketones and aldehydes 775
Chlorides see “Halides”
Chlorides acyl, and leaving groups 449
Chlorides acyl, from aldehydes 914
Chlorides acyl, reduction to aldehydes 533
Chlorides alkyl, by sodium salt of alkyl sulfonic acids 944
Chlorides aryl, by the Sandmeyer reaction 936
Chlorides aryl, from aryl diazonium salts 935
Chlorides vinyl from dichlorides 1195
Chlorides vinyl, from 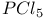 with aldehydes or ketones 1195 with aldehydes or ketones 1195
Chlorine photochemical cleavage 245
Chlorine reaction with aldehydes 914
Chlorine reaction with alkenes 1042
Chlorine reaction with aromatic compounds 704
Chlorine reaction with hydrocarbons 907—911
Chlorine reaction with hydrocarbons and 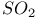 924 924
Chloro esters see “Esters chloro”
Chloro ketones and Favorskii rearrangement 1403
Chloro ketones rearrangement with base 1403
Chloroacetic acid, thermodynamic ionization values 350
Chloroallene 1400
Chloroamides, conversion to  -lactams 514 -lactams 514
Chloroamines, from alkenes 1045
Chloroamines, from amines 819
Chloroarrilines, by benzyne intermediates 860
Chlorobenzene reaction with amide bases 854
Chlorobenzene resonance structures 41
Chloroethane, bond polarization 16
Chloroform hydrolysis of 464
Chloroform reaction with amines 506
Chloroform reaction with phenoxide 716
Chloroformates, alkyl, reduction of 529
Chlorohydrins, from alkenes 1043
Chloromalic acid 150
Chloromethane, reaction with chloride ion, energy 394
Chloromethylation, of aromatic compounds 721
Chloroqumones, vinyl substitution 429
Chlorosuccinic acid 391
Chlorosulfenation 924
Chlorosulfites, alkyl, reaction with carboxylic acid salts 489
Chlorosulfonation, of hydrocarbons 924
Chlorosulfonyl isocyanate, reaction with alkenes 1251
Chlorosulfonyl isocyanate, reaction with allenes 1251
Chlorosulfonyl isocyanate, reaction with cyclopropenes 1251
Chlorosulfonyl isocyanate, reaction with dienes 1251
Chlorosulfonyl isocyanate, reaction with imines 1251
Chlorosulfuric acid, reaction with aromatic compounds 703
Chlorotrimethylsilane and hydrogenolysis of alcohols 527
Chlorotrimethylsilane and the acylohi condensation 1562
Chlorotrimethylsilane, reaction with alkenes 992
Chlorotris (triphenylphosphine)rhodium 1339
Cholest-6-en-3-one 377
Cholesta-3,5-diene 320
Cholic acid, and resolution 152
Chromatography and resolntion 152
Chromatography, gas and resolution 156
Chromic acid and oxidation of alcohols 1515
Chromic acid, oxidation of hydrocarbons 915
Chromium reagents Jones reagent 1514
Chromium reagents oxidation of alcohols 1514
Chromium reagents, Collins’ reagent 1514
Chromium trioxide, oxidation of primary alcohols 1537
Chromium trioxide, oxidative cleavage of alkenes 1525
Chromium trioxide, oxidative cleavage of ketones 1521
Chromium trioxide, reaction with polycyclic aromatics 1535
Chromophore and conjugation 310
Chromophore, definition 308
Chromyl chloride and the Etard reaction 1534
Chromyl chloride oxidation of methyl aryls 1534
Chugaev reaction 1330
CIDNP and aromatic radical substitution 898
CIDNP and coupling of organometallics with RX 538
CIDNP and enantiotopic atoms 166
CIDNP and formation of Grignard reagents 806
CIDNP and hydroformylation of alkenes 1037
CIDNP and intermediates 288
CIDNP and NMR 240—241
CIDNP and radicals 240—241
CIDNP and SET mechanisms 403
CIDNP and the Stevens rearrangement 1420
CIDNP in reactions of aromatic compounds and peroxides 932
CIDNP in reactions of organ olithium reagents and alkyl halides 808
CIDNP, andcarbenes 791
Cieplak effect 988
Cinchona alkaloids, and dihydroxylation of alkenes 1050
Cine substitution 854 864
CIP method, and alkenes 157
CIP rulesand diastereotopic atoms 166
CIP system 139—141
CIP system and Fischer projections 141
Circular dichroism, and enantiomeric composition 143
Circulene 44
Circumambulatory rearrangements 1440
cis,trans-2,4-Hexadiene 1427
cis-2-Bromostilbene 1301
cis-2-Butene 290
cis-3,4-Dichlorocyclobntene 1434
cis-3,4-Dimethylcyclobutene 1427
cis-l,3-Pentadiene, resonance energy 37
Cis/trans isomerization, and carbenes 249
Cis/trans isomers 156—163 (also see “Alkenes” “Isomers”)
Cis/trans isomers and alkenes 157—160
Cis/trans isomers and betweenanenes 186
Cis/trans isomers and bridged compounds 161—163
Cis/trans isomers and C=X units 157—158
Cis/trans isomers and captodative compounds 159
Cis/trans isomers and carbenes 248
Cis/trans isomers and diastereomers 157—160
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



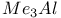

 and alcohols
and alcohols 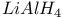

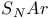 reactions
reactions  -bond rearrangements
-bond rearrangements  with aldehydes or ketones
with aldehydes or ketones 
 -lactams
-lactams