|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Acetals from ortho esters 1642
Acetals from phenols 1642
Acetals hydrogenolysis, reagents for 528
Acetals hydrolysis 465
Acetals hydrolysis and the  mechanism 466 mechanism 466
Acetals hydrolysis and the Bronsted coefficient 466
Acetals hydrolysis, and microwaves 467
Acetals hydrolysis, reagents for 467
Acetals ketene, reaction with aldehydes or ketones 1249
Acetals ketene, silyl, and the Mukaiyama aldol reaction 1223
Acetals rate of hydrolysis and structure 466
Acetals reaction with carboxylic acids 489
Acetals reaction with Lewis acids 467
Acetamides, N-chloro, rearrangement of 728
Acetates allylic, coupling with carboxylic acids 944
Acetates coupling with nickel tetracarbonyl 545
Acetates elimination with Pd 1330
Acetates reaction with organocuprates 545
Acetic acid and acid strength 327
Acetic acid pKa 342
Acetic acid reaction with alkenes and 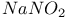 1339 1339
Acetic acid thermodynamic ionization values 350
Acetic anhydride and DMSO, for oxidation of alcohols 1516
Acetic anhydride cleavage of ethers 489
Acetic anhydride dehydrating agent 491
Acetic anhydride reaction with alkenes 1058
Acetic anhydride reaction with oximes 1348
Acetic-phosphoric anhydride, acylation of alcohols 483
Acetoacetic ester enol of 74—75
Acetoacetic ester synthesis 549
Acetoacetic esters alkylation 549
Acetoacetic esters dianion 550
Acetolysis, substituent effects 412
Acetone, as a solvent 450
Acetonitrile as a solvent 450
Acetonitrile reaction with hydrocarbons 925
Acetyl hypofluorite, and aromatic fluorination 707
Acetyl tosylate, cleavage of ethers 490
acetylene see “Ethyne”
Acetylene dicarboxy, reaction with bromine 973
Acetylene reaction with aromatic compounds 708
Acetylides see “Alkyne anions”
Achirality, definition 125
Aci form nitronic acids 76
Aci form of nitro compounds 76
Acid derivatives, reaction with amines 512
Acid derivatives, reaction with Grignard reagents 567
Acid derivatives, reactivity and leaving groups 449
Acid form, azinic acids 76
Acid strength ab initio calculation 328
Acid strength acetic acid 342
Acid strength alcohols vs. carboxylic acids 344
Acid strength alcohols, order of 350
Acid strength amides 344
Acid strength amines 344
Acid strength and conformation 347
Acid strength and diazonium salts coupling 700
Acid strength and entropy effect 347
Acid strength and equilibrium constants 336
Acid strength and free energy 336
Acid strength and Ho 334—335
Acid strength and hybridization 348—349
Acid strength and hydrogen bonding 99 101—102 346
Acid strength and hydron donors 352
Acid strength and inductive effects 17
Acid strength and internal hydrogen bonding 333
Acid strength and lyonium ions 335
Acid strength and principle of imperfect synchronization 334
Acid strength and reaction medium 349—351
Acid strength and reaction with metals 793
Acid strength and solvation 349
Acid strength and steric effects 346
Acid strength and structure 342—349
Acid strength and the Hammett reaction constant 350
Acid strength and the Periodic table 345
Acid strength C-H, and hybridization 433
Acid strength crystallographic scale 328
Acid strength definition 327
Acid strength esters 344
Acid strength ethers 344
Acid strength field effects 342—343
Acid strength gas phase 350
Acid strength Hammet acidity function 334
Acid strength hydroxybenzoic acids 346
Acid strength kinetic 228—229
Acid strength Lewis acids 340
Acid strength nitroacetic acid 342
Acid strength of  -hydrogen in E2 elimination 1316 -hydrogen in E2 elimination 1316
Acid strength of 1,3-pentanedione 347
Acid strength of dimethyl malonate 347
Acid strength of dinitromethane 230
Acid strength of fluorene 52
Acid strength of indene 52
Acid strength of Meldrum’s acid 347
Acid strength of nitromethane 230
Acid strength of pentakis (trifluoromethy) clopentadiene 52
Acid strength of quinoline conjugate acid 347
Acid strength of solvents 334—336
Acid strength of squaric acid 70
Acid strength phenols 345
Acid strength resonance effects 343—345
Acid strength scales of acidity 335
Acid strength statistical effects 345—346
Acid strength t-butylbenzoic acids 346
Acid strength table 329—331
Acid strength table of acidity constants 343
Acid strength the Bunnett-Olsen equation 335
Acid strength thermodynamic 228
Acid strength tri(pentafluorophenyl)methane 343
Acid strength tricyanomethane 344
Acid strength triphenylacetic acid 343
Acid, definition 333
Acid, formic see “Formic acid”
Acid, salts see “Carboxylic acid salts”
Acidity constants 327—328 333 343
Acidity scales 335
acids also see “Carboxylic acids”
Acids addition to ketenes 992
Acids, and Bronsted theory 327—328
Acids, and catalysis 336—338
Acids, as proton donors 327
Acids, carboxylic see “Carboxylic acids
Acids, hard and soft, definition 340
Acids, hardness, table 340
Acids, Lewis see “Lewis acids”
Acids, mineral, reaction with alcohols 518
Acids, peroxy see “Peroxyacids”
Acids, reaction with alkenes 991
Acids, reaction with alkynes 975
Acids, reaction with cyclopropanes 989
Acids, reaction with organometalhc compounds 794
Acids, super acids 328
Acids, thio see “Thioacids”
Acrolein, reaction with borane 1031
Acrylonitrile, conjugate addition to 976
Actinometer, definition 322
Activated complex, and transition states 279
Activating groups and electrophilic aromatic substitution 681—684
Activating groups and Friedel-Crafts alkylation 709
Activating groups and the Ullmann reaction 871
Activating groups, chair-to-chair interconversions 173
Activating groups, electrophilic aromatic substitution, multiple substituents 687
Activating groups, for 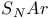 reactions 858 reactions 858
Activation free energy and reaction coordinate 279
Activation free energy of 278—279
Activation hardness, and electrophilic aromatic substitution 692
Activation volume, and high pressure reactions 457—458
Active hydrogen compounds and the Knoevenagel reaction 1225—1228
Active hydrogen compounds base catalyst condensation 1219
Active hydrogen compounds Michael addition to alkenes 1022—1024
| Active hydrogen compounds reaction with aldehydes or ketones 1225—1228
Active hydrogen compounds reaction with aryl halides 869
Acyl anions, and Umpolung 553
Acyl azides see “Azides”
Acyl azides from acyl halides 515
Acyl bromides, from aldehydes 914
Acyl carbon, and leaving groups 448
Acyl cations 223; see “Acylium ion”
Acyl chlorides see “Chlorides acyl”
Acyl cyanides, from acyl halides 573
Acyl fluorides from acyl chlorides and HF 524
Acyl fluorides from anhydrides and HF 524
Acyl fluorides from DAST 524
Acyl fluoroborates, reaction with alkenes 1058
Acyl halides see “Halides acyl”
Acyl hydrazides, conversion to esters 488
Acylals from acylation of ketones 1642
Acylals from addition of carboxylic acids to alkynes 1642
Acylals from aldehydes and anhydrides 1245
Acylals from bisdecarboxylation of malonic acids 1642
Acylals from oxidation of arylmethanes with 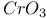 and and 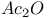 1642 1642
Acylals, from acylation of aldehydes 1642
Acylation 569
Acylation Friedel-Crafts 712—714
Acylation of alkenes 784
Acylation of amines 511
Acylation of amino acids 812
Acylation of aromatic rings 710
Acylation of conjugated dienes 784
Acylation of enamines 788
Acylation of esters 569—570
Acylation of ketones 569
Acylation reductive, of ketones 1182
Acylation with aldehydes, of heterocycles 934
Acylhydrazides, reaction with nitrous acid 1412
Acylium ion, reaction with alkenes 784
Acyloin condensation 1562
Acyloin condensation with tethered diesters 1602 (ref. 748)
Acyloins also see “Hydroxy 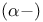 ketones” ketones”
Acyloins, and the benzoin condensation 1243
Acyloxy amides by the Passerini reaction 1252
Acyloxylation of alkenes 997 1033 1048 1058
Acyloxylation of hydrocarbons 923
Acyloxylation of ketones, reagents for 923
Acylureas, for Hofmann rearrangement 1411
Adamantanes 1396
Adamantanes and optical activity 131
Adamantanes, cleavage during hydrogenation 815
Adamantanones 1173
Adamantyl cation 224—225 1383
Adamantyl compounds, and solvent ionizing power 452
Addition 1,2vs. 1,4 980
Addition 1,4 see “Addition conjugate”
Addition compounds 102—114
Addition compounds, definition 102
Addition reactions, mechanism 276
Addition reactions, of nitrenes 254
Addition reactions, of radicals 246
Addition reactions, to cyclopropanes 988—990
Addition to alkenes, and antiaromaticity 983
Addition to alkenes, and hyperconjugation 987
Addition to alkenes, and the Cieplak effect 988
Addition to alkenes, by alkanes 1017—1019
Addition to alkenes, by boranes 1012—1016
Addition to alkenes, cyclic mechanism 979
Addition to alkenes, electronic effects 987
Addition to alkenes, exo/endo diastereoselectivity 987
Addition to alkenes, field effects 987
Addition to alkenes, reactivity of alkenes 981
Addition to alkenes, regioselectivity 984
Addition to alkynes 997
Addition, alcohols to alkenes 996
Addition, alkenes to alkenes, and Ziegler catalysts 1020
Addition, conjugate 979—981
Addition, conjugate and 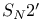 reactions 980 reactions 980
Addition, conjugate and chiral templates 1029
Addition, conjugate and enolate anions 1023
Addition, conjugate and Grignard reagents 1028
Addition, conjugate and Mannich bases 1023
Addition, conjugate and organolithium reagents 1029
Addition, conjugate chiral additives 1023
Addition, conjugate cyanoethylation 976
Addition, conjugate definition 976
Addition, conjugate enantio selectivity of 1029
Addition, conjugate hydrogenation of dienes 1005
Addition, conjugate Michael addition 976
Addition, conjugate organocuprates 1027
Addition, conjugate reduction 1008—1009
Addition, conjugate with nucleophiles 1022—1024
Addition, conjugate with radicals 1024
Addition, conjugate, alkyl halides to dienes 1047
Addition, conjugate, amines to alkenes 1000
Addition, electrophilic 276
Addition, electrophilic to alkenes 970—975
Addition, electrophilic to alkenes, diastereoselectivity 971
Addition, electrophilic to cyclopropanes 988—990
Addition, Michael 977 1022—1024
Addition, nucleophilic 276
Addition, nucleophilic and conjugation 1174
Addition, nucleophilic and iminium ions 1178
Addition, nucleophilic and steric hindrance 1174
Addition, nucleophilic to alkenes 1000
Addition, nucleophilic to carbonyls 1173
Addition, pericyclic 276
Addition, radical 276
Addition, radical intramolecnlar 985
Addition, radical reversibility 981
Addition, radical to alkenes 977—979
Addition, radical to alkenes, and cis/trans isomerization 979
Addition, radical to alkenes, and ESR 979
Addition, radical to alkenes, and the Felkin-Ahn rule 981
Addition, radical to alkenes, by silane tethers 1032
Addition, radical to alkenes, stereo selectivity 978
Addition, radical to alkenes, steric effects 985
Addition, radical to conjugated compounds 1032
Addition, radical to cyclopropanes 990
Addition, radical to dienes 978
Addition, termolecular 972
Addition-elimination mechanism and oxidation-reduction 1509
Addition-elimination mechanism and substitution at vinyl carbon 428
Addition-elimination mechanism and transmetallation 803
Addition-elimination mechanism metal hydride 772
Additions, IUPAC rules 383
Adsorption, and catalytic hydrogenation 1006
Aggregates and enolate anions 237
Aggregates and organolithium reagents 237
Aggregates in dialkylamide bases 348
Aggregation, effect of HMPA 349
AIBN and formation of aryl esters 870
AIBN and hydrogenolysis of alcohols 527
AIBN and radical addition of silanes to alkenes 1039
AIBN and radical coupling of dialdehydes 1561
AIBN and radical cyclization to C=N and C=O 1244
AIBN and reaction of aldehydes with ammonia/NBS 925
AIBN and the Barton-McCombie reaction 527
AIBN conpling silanes and lactones 536
AIBN radical initiator 911 914
Alanes, vinyl 1210
Alcohols acylation with acetic-phosphoric anhydride 483
Alcohols and acid strength 344
Alcohols and Birch reduction 1010
Alcohols and Oppenauer oxidation 1516
Alcohols and Swern oxidation 1516
Alcohols and the Dess-martin periodinane 1516
Alcohols and the Koch-Haaf reaction 564
Alcohols and the Meerwein-Ponndorf-Verley reduction 1199
Alcohols and the Oppenauer oxidation 1199
Alcohols and the Pinner synthesis 1183
Alcohols by Payne rearrangement 1643
Alcohols by radical oxygenation 914
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 mechanism
mechanism 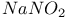
 -hydrogen in E2 elimination
-hydrogen in E2 elimination 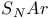 reactions
reactions 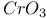 and
and 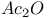
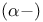 ketones”
ketones”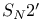 reactions
reactions