|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Azo compounds hydroxy, by the Wallach rearrangement 1464
Azo compounds hydroxy, from azoxy compound 1464
Azo compounds isomerization of 320 700
Azo compounds reduction, reagents for 1556 1559
Azo compounds thermal cleavage 245
Azo dyes, by diazonium coupling 700
Azo-Cope rearrangements 1452
Azobenzenes and nitrenes 254
Azobenzenes from aromatic nitro compounds 1553
Azodicarboxylate, diethyl see “Diethyl azodicarboxylate”
Azomethine imines, as 1,3-dipoles 1060
Azomethine ylids, as 1,3-dipoles 1060
Azoto-isobutyronitrile see “AIBN”
Azoxy compounds, as 1,3-dipoles 1060
Azoxy compounds, from alkanediazotates 1659
Azoxy compounds, from alkyl halides 516 1659
Azoxy compounds, from aromatic nitro compounds 1563
Azoxy compounds, from hydroxylamines 819 1659
Azoxy compounds, from reduction of hydroxylamines 1659
Azoxy compounds, from reduction of nitro compounds 1659
Azoxy compounds, from reduction of nitroso compounds 1659
Azoxy compounds, fromnitroso compounds 819 1659
Azoxy compounds, rearrangement with acid 1464
Azoxy compounds, reduction with 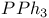 1557 1557
Azoxy compounds, reduction with Zn-HCl 1559
Azoxybenzenes, from aromatic nitro compounds 1553
Azulene and aromaticity 54
Azulene photochemistry 315
Azulenyl nitrones, spin traps 239
B orates, in borane reduction of aldehydes or ketones 1200
B strain see “Strain”
Bacillus stearothermophilus, and oxidation of alcohols 1517
Back strain see “Strain”
Backside attack, and  390 390
Bacterial monooxygenases, asymmetric oxidation 1590 (ref. 457)
Baeyer test, for alkenes 1049
Baeyer — Villiger rearrangement 1417—1418
Baeyer — Villiger rearrangement and the Dakin reaction 1528
Baeyer — Villiger rearrangement in competition with epoxidation 1052
Bakelite polymers 720
Baker — Nathan effect 72
Baker — Nathan order and electrophilic aromatic substitution 685
Baker — Nathan order and nucleophilic substitution 437
Bakers yeast and reduction of alkenes 1004
Bakers yeast and reduction of aromatic nitro compounds 1553
Bakers yeast and reduction of azides 1555
Bakers yeast and reduction of ketones 1200
Bakers yeast and reduction of oximes 1554
Baldwin’s rules 282—284
Baldwin’s rules and radical cyclization 978 986 1040
Balz — Schiemann reaction 875
Bamberger rearrangement 878
Bamford — Steven reaction 1335
Barbaralane 1448
Barbier reaction 1205 1210
Barbier — Wieland procedure 1526
Barbituric acid 514
Barrelene 136 1502
Bartell MUB-2 force field calculations 179
Barton reaction 1463
Barton — McCombie reaction 527
Base catalyzed reactions 337
Base strength amines 346 349
Base strength and F strain 346
Base strength and face strain 346
Base strength and hybridization 348—349
Base strength and reaction medium 349—351
Base strength and resonance effects 344
Base strength and solvation 349
Base strength and steric effects 347
Base strength and structure 342—349
Base strength definition 327
Base strength entropy effect 347
Base strength hydrogen bond scale 332
Base strength table 332
Bases and Bronsted theory 327—38
Bases and catalysis 336—338
Bases as proton acceptors 327
Bases dialkylamides, and structure 348
Bases for formation of ketone enolate anions 551
Bases for preparing alkyne anions 561
Bases formation of enolate anions 548
Bases hard and soft 340
Bases hardness, table 340
Bases HMPA and aggregation effects 349
Bases proton sponges 347
Bases reaction with non — enolizable ketones 814
Basicity ambident nucleophiles 460
Basicity and ambident nucleophiles 460
Basicity and carbanions 227—228
Basicity and hydrogen bonding 99
Basicity and leaving group ability 445
Basis sets, for the Diels — Alder reaction 1070
Basketane 1502 (ref. 655)
Baylis — Hillman reaction 1212
Bcnzcnediazonium chloride 816
Beckmann rearrangement 1349 1381 1384 1415—1416
Beckmann rearrangement and the Neber rearrangement 1410
Beckmann rearrangement and the Ritter reaction 1416
Beckmann rearrangement and the Schmidt rearrangement 1416
Beckmann rearrangement migratory aptitudes 1415
Beilstein 1605 1614
Benedict’s solution, oxidation of aldehydes 917
Benkeser reduction 1012
Bent bonds, and cyclopropane 181
Benz (ft)isoquinoline, and Chemical Abstracts 1613
Benzamide, hydrolysis of 427
Benzene canonical forms 32
Benzene derivatives see “Aromatic compounds”
Benzene Dewar 32 1083 1090 1433 1449
Benzene dihydroxylation of 1050
Benzene electron donor — acceptor complexes 103
Benzene electrophilic aromatic substitution, product distributions 693
Benzene from acetylene 1089
Benzene from cyclohexane 1510
Benzene heat of atomization 35
Benzene heat of hydrogenation 35
Benzene MO, and electrophilic aromatic substitution 683
Benzene molecular orbitals 33
Benzene multiple substituents, and electrophilic aromatic substitution 687
Benzene oxide, by Cope rearrangement 1448
Benzene ozonolysis of 1523
Benzene phenonium ion from, charge distribution 685
Benzene reaction with alkenes 1093
Benzene reaction with carbenes 1087
Benzene reaction with Pseudomonas putida 1050
Benzene resonance energy 35 49
Benzene UV spectra 311
Benzene valence bond isomers 1084
Benzenonium ion 678
Benzenonium ion charge distribution 685
Benzenonium ion from diazonium salts 853
Benzhydryl chloride 396
Benzhydryl p — toluenesulfonate, hydrolysis of 465
Benzidine rearrangement 1455—1456
Benzidine rearrangement and semidines 1455
Benzidine rearrangement and sigmatropic rearrangements 1456
Benzil 1403
Benzil reaction with Grignard reagents 1208
Benzil-benzylic acid rearrangement 1403
Benzocyclobutene 45
Benzocycloheptatrienyl anion, and antiaromaticity 62
Benzocyclopropene 45 186
Benzofurans, from cyclization with low valent titanium 1562
Benzoic acid derivatives see “Carboxylic acids aryl”
Benzoic acid, from toluene 1527
Benzoin condensation 1240 1243
Benzoins 1243 1403
Benzophenone, and photochemistry 321
Benzophenone-4-carboxylic acid 1532
| Benzothiazoles, and reaction with organolithium reagents 1029
Benzvalene 1449
Benzyl alcohols reaction with phosgene 483
Benzyl alcohols substitution with chloride ion 457
Benzyl chloroformates, from amines 507
Benzyl ethers, selective cleavage 520
Benzyl intermediates, energy levels 56
Benzylammonium salts, reaction with sodium amide 877
Benzylic acid 1403
Benzylic cations 222 (also see “Carbocations”)
Benzylic radicals 902
Benzynes 854
Benzynes and ElcB elimination 1310
Benzynes and the Diels — Alder reaction 855
Benzynes in the Diels — Alder reaction 1062
Benzynes mechanism 864
Benzynes mechanism, substrate effects 859
Benzynes reaction with anthracene 1063
Benzynes resonance contributors 855
Bergman cyclization 1432 1447
Betaines and the Wittig reaction 1234
Betaines from epoxides and phosphines 1235
Betaines from homologation of aldehydes and ketones 1407
Betweenanenes 186
Biaryls by the Gomberg reaction 928
Biaryls by the Gomberg — Bachmann reaction 928
Biaryls by the Scholl reaction 711
Biaryls by the Ullman reaction 870
Biaryls from aromatic compounds 933
Biaryls from aryl diazonium salts 928 937
Biaryls from aryl halides 870 933
Biaryls preparation of 711
Bicyclic compounds and cis/trans isomers 162—163
Bicyclic compounds and memory effects 1386
Bicyclic compounds and the  reaction 397 764 reaction 397 764
Bicyclic compounds solvolysis 1386
Bicyclobntanes and  -bond rearrangements 1460 -bond rearrangements 1460
Bicyclobntanes reaction with silver tetrafluoroborate 1460
Bicyclobutane 183
Bicyclohexenes 183
Bicyclopentanes 182—183
Bicyclopropenyl 1449
Bicyclopropyl systems 1459
Bicyclopropyls 1085
Bicyclo[2.2.1]hept-2,3-en-7-ones, heating 1347
Bicyclo[2.2.1]hept-2-ene see “norbornene
Bicyclo[2.2.2] compounds, and E2elimination 1303
Bicyclo[2.2.2]octylmethyl tosylate 437
Bicyclo[3.1.0]hexyl-6-tosylate, exo- 1435
Bicyclo[3.2.0]hept-2-en-6-yl acetate, endo- 1442
Bicyclo[3.2.0]heptane 162
Bicyclo[3.2.2]non-l-ene 188
Bicyclo[4.1.0]hexan-4-one, reaction with 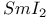 1408 1408
Bicyclo[4.2.1]non-l(8)ene 188
Bicyclo[4.2.2]dec-3-ene 188
Bifurcated hydrogen bonds 99
Bimolecular mechanism 759—763
Bimolecular,  mechanism 390 mechanism 390
BINAP 1267 (ref. 321)
BINAP and reaction of boranes with conjugated ketones 1032
BINAP and reduction of aldehydes and ketones 1201
Binaphthyl 712
BINOL, and reaction of epoxides with ammonia 504
Biochemical processes, and resolution 153
Biosynthesis, lanosterol 1019
Biphenylene 1077
Biphenylenediol, and hydrogen bonding 100
Biphenyls and atropisomerism 132
Biphenyls and optical activity 131—132
Biphenyls diamino 1455
Biradicals see “Radicals”
Birch and alkenes 1008
Birch reduction 1010—1011
Bis amides, by the Ugi reaction 1252
Bis(2-methoxyethoxy)aluminum hydride, sodium see “Red-Al”
Bis(diphenylphosphino)ethane see “dppe
Bis(picolinato)iron (II) 1532
Bis(s-collidine)iodine (I) tetrafluoroborate 1539
Bisamides 1187
Bischler — Napieralski reaction 721
Bismuth trichloride, and the Knoevenagel reaction 1226
Bisperoxides, and ozonolysis 1523
Bisphenol, by hydroxyalkylation 719
Bisulfite addition products 1185
Bisulfite addition products reaction with cyanide 1240
Bisulfite, reaction with aldehydes or ketones 1185
Blaise reaction 1213
Boat conformation 172
Boat conformation and the Cope rearrangement 1446
Boat conformation fused ring aromatics 45
Boat conformation in benzene derivatives 44
Boekelheide reaction 1421
Bond angles 21—22
Bond angles and E2 elimination 1303
Bond angles and hybridization 21
Bond angles and radical abstraction 903
Bond angles in strained molecules 21
Bond angles table 21
Bond dissociation energy and radicals 900
Bond dissociation energy HX and RX 911
Bond distance 18—21 (also see “Bonding”)
Bond distances in benzenediazonium chloride 816
Bond energy 24; see “Energy bond
Bond length and cross-conjugation 40
Bond length and cross-conjugation and strain 184
Bond length, and cross-conjugation picryl iodide 43
Bond moments 15—16
Bond order benzene 34
Bond order naphthalene 49
Bond order valence bond, and Birch reduction 1011
Bond polarization 16—18
Bond rotation and NMR 295
Bond rotation and temperature 295
Bonding  45 45
Bonding  9 9
Bonding and bent bonds 181
Bonding and double bonds 9
Bonding and influence of Si on bond distance 19
Bonding and radicals 244
Bonding and van de Waals forces 98
Bonding bond distance 18—21
Bonding bond distances, table 19 20
Bonding bond length and bond energy 24
Bonding bond length, butadiene 37
Bonding charge — transfer bonding 104
Bonding coordinate covalent 13
Bonding covalent 3—6 13
Bonding delocalized 32
Bonding delocalized, molecule type 36—39
Bonding hydrogen see “Hydrogen bonding”
Bonding in benzene 32—34
Bonding in cyclopropane 181
Bonding in diatomic nitrogen 11
Bonding in electron donor — acceptor complexes 103
Bonding in ethene 8—9
Bonding in Grignard reagents 234
Bonding in methane 8 10
Bonding in triple bonds 9
Bonding inethyne 9—10
Bonding ionic 15
Bonding multiple 8—10
Bonds axial 172
Bonds conjugated, in butadiene 36
Bonds energy 312
Bonds equatorial 172
Bonds heterolytic cleavage 274
Bonds homolytic cleavage 275
Bonds rotation and conformation 167—178
Boolean operator 1633
Boord reaction 1344
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



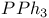

 reaction
reaction  -bond rearrangements
-bond rearrangements