|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Esters dissolving metal reduction of 529
Esters dithiocarboxylic, from carboxylic acids 1184
Esters enolate anion, reaction with esters 569—570
Esters enolates also see “Enolate anions”
Esters formate, reaction with alkenes 1034
Esters formation from carboxylic acids and microwaves 484
Esters formation of and steric hindrance 485
Esters formation, mechanism of 485
Esters from  -keto esters 1662 -keto esters 1662
Esters from 1,1,1-trihalides 463
Esters from acetophenones 1663
Esters from acyl halides 483 1662
Esters from acyl hydrazides 488
Esters from acyl peroxides 1662
Esters from alcohols 483 486 488 801 1183 1662 1663
Esters from alcoholysis of acyl halides 482
Esters from aldehydes 1565 1663
Esters from alkenes 997 1662 1663
Esters from alkyl halides 564 1662
Esters from alkyne ethers 1662
Esters from amides 488 1662
Esters from anhydrides 483 1662
Esters from aryl esters 1662
Esters from aryl halides 1662
Esters from aryl nitro compounds 1662
Esters from boranes 1662
Esters from carboxylic acid salts 1662
Esters from carboxylic acids 484—486 489 490 997 1528 1662 1663
Esters from chloro ketones 1403
Esters from chloroformates 1662
Esters from conjugated esters 1662
Esters from cyclic ketones 1663
Esters from diazo compounds 490 1662
Esters from diazo esters 1662
Esters from diazo ketones 1406
Esters from dimethylfonnamide acetals 489
Esters from enol ethers 1539 1663
Esters from esters 486 1662
Esters from ethers 483 1534 1662 1663
Esters from Grignard reagents 1662
Esters from halo esters 1662
Esters from hydrazides 1662
Esters from hydrocarbons 922—923
Esters from hydrolysis of ortho esters 1662
Esters from iminium salts 1183
Esters from ketenes 1662
Esters from keto esters 812
Esters from ketones 1417 1663
Esters from mercury hydrides 1662
Esters from methyl ketones 1662
Esters from nitriles 1183 1663
Esters from nitrogen heterocycles 1662
Esters from organometallic compounds 1662
Esters from oxazines 559 1662
Esters from oxidative cleavage of silyl ketones 1521
Esters from ozonides 1663
Esters from phenols 1662
Esters from rearrangement of  -halo ketones 1663 -halo ketones 1663
Esters from rearrangement of diazo ketones 1663
Esters from tin hydrides 1662
Esters from transesterification 1662
Esters from trihalides 1662
Esters from unsaturated esters 1662
Esters from unsaturated ketones 1662
Esters gas phase reactions 473
Esters glycidic 1230
Esters homologation of 568
Esters hydrogenolysis of 527 529
Esters methyl, cleavage with NaCN-HMPA 562
Esters methyl, from diazomethane and carboxylic acids 490
Esters Mosher’s derivative 142—143
Esters of glutaric acid and the Stobbe condensation 1224
Esters of inorganic acids, hydrolysis of 464
Esters oxime and the Beckmann rearrangement 1415
Esters phenolic, from arylmethyl ketones 1528
Esters phenylthio, radical cyclization 1244
Esters phosphonate and Lawesson’s reagent 1331
Esters phosphonate, cleavage 1331
Esters propargyl, reaction with organocuprates 545
Esters pyrolysis of 1327 1329
Esters reaction with 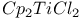 1552 1552
Esters reaction with 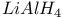 1551 1551
Esters reaction with 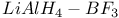 1550 1550
Esters reaction with 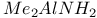 1195 1195
Esters reaction with 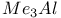 567 567
Esters reaction with alcohols 486
Esters reaction with aldehydes or ketones 1223
Esters reaction with amide bases 1224
Esters reaction with amines 510
Esters reaction with ammonia 510
Esters reaction with carboxylic acid dianions 572
Esters reaction with carboxylic acids 491
Esters reaction with dimagnesium compounds 1214
Esters reaction with Grignard reagents 567 1214
Esters reaction with ketone enolate anions 571
Esters reaction with Lawesson’s reagent 1184
Esters reaction with Li and amines 529
Esters reaction with lithium amides 510
Esters reaction with lithium iodide 521
Esters reaction with nitrile enolate anions 572
Esters reaction with nitrous acid 465
Esters reaction with organolithium reagents 567
Esters reduction by the Bouveault — Blanc procedure 1551
Esters reduction to alcohols 1551
Esters reduction to ethers 1550
Esters sterically hindered, and hydrolysis 470
Esters substitution with bromo esters and Zn 1213
Esters sulfenylation of 783
Esters tethered, acyloin condensation 1602 (ref. 748)
Esters thio see “Thioesters”
Esters xanthate and the Chugaev reaction 1330
Esters xanthate, pyrolysis of 1330
Esters, acetoacetic see “Acetoacetic esters”
Esters, conjugated and Michael reactions 1022—1024
Esters, conjugated from esters and aldehydes or ketones 1223
Esters, conjugated reaction with aldehydes 1212
Esters, conjugated reaction with organocuprates 1027
Esters, hydrolysis 371 469—474 1050
Esters, hydrolysis and freezing point depression 472
Esters, hydrolysis and labeling 290 425 472
Esters, hydrolysis and microwaves 470
Esters, hydrolysis and phase transfer catalysis 470
Esters, hydrolysis and ring strain 473
Esters, hydrolysis and stereochemistry 472
Esters, hydrolysis and the tetrahedral mechanism 425
Esters, hydrolysis enzymatic 470
Esters, hydrolysis isotope effects 472
Esters, hydrolysis mechanism 470—473
Esters, hydrolysis reagents for 470
Esters, imino and the Chapman rearrangement 1464
Esters, imino thermolysis 1464
Esters, inorganic alkylation with 478
Esters, inorganic from alcohols 1676
Esters, inorganic from alkenes 1677
Esters, inorganic from alkyl halides 1676
Esters, inorganic from aryl halides 1676
Esters, inorganic from diazonium salts 1677
Esters, inorganic from inorganic halides 1676
Esters, inorganic from trialkylboranes 1676
Esters, inorganic preparation of 493
Esters, malonic see “Malonic esters”
Esters, sulfonate alkylation with alkoxides 478
Esters, sulfonate allylic coupling with nickel tetracarbonyl 543
Esters, sulfonate as leaving groups 446
Esters, sulfonate by the Knoevenagel condensation 1687
Esters, sulfonate coupling with Grignard reagents 543
Esters, sulfonate coupling with organocuprates 543
Esters, sulfonate elimination reactions 1340
Esters, sulfonate elimination with base 1330
Esters, sulfonate from alcohols 1687
| Esters, sulfonate from aldehydes 1687
Esters, sulfonate from aryl nitro compounds 1687
Esters, sulfonate from boranes 1687
Esters, sulfonate from ethers 494 1687
Esters, sulfonate from halo sulfonic acid esters 1687
Esters, sulfonate from ketones 1687
Esters, sulfonate from sulfonamides 576
Esters, sulfonate from sulfonic acid derivatives 494 576 1687
Esters, sulfonate hydrolysis 465
Esters, sulfonate hydrolysis and labeling 574
Esters, sulfonate hydrolysis of 465 575
Esters, sulfonate of phenols, reduction of 867
Esters, sulfonate reaction with 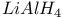 526 526
Esters, sulfonate reaction with Grignard reagents 574
Esters, sulfonate reaction with halide ions 518
Esters, sulfonate reagents for hydrogenolysis 526
Etard reaction 1534
Ethane cleavage and entropy 278
Ethane conformational energy diagram 168
Ethane heat of combustion 22
Ethanoic acid see “Acetic acid”
Ethanol, with sodium, for reduction of aldehydes or ketones 1199
Ethanolamine and dealkylation of ammonium salts 503
Ethanonium ion 770
Ethene and resonance 41
Ethene bond angles 9
Ethene bonding 8—9 12
Ethene reaction with bromine 973
Ethene valence electrons 12
Etherification, transannular 920
Ethers  -halo, and the Boord reaction 1344 -halo, and the Boord reaction 1344
Ethers allyl aryl, and Claisen rearrangement 1449
Ethers allyl vinyl, and Claisen rearrangement 1452
Ethers allyl vinyl, and [l,3]-alkyl sigmatropic rearrangements 1442
Ethers allylic, anion, and the Wittig rearrangement 558
Ethers allylic, carbanions and [2,3] Wittig rearrangement 1454
Ethers allylic, reaction with Grignard reagents 546
Ethers allylic, reaction with organolithium reagents 558
Ethers and acidity 344
Ethers and dialkylamides basses 349
Ethers and esterification reactions 486
Ethers aryl and the 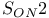 mechanism 862 mechanism 862
Ethers aryl, by the Ullmann ether synthesis 863
Ethers aryl, deoxygenation 734
Ethers aryl, from aryl halides 862
Ethers as solvents for Grignard reactions 546
Ethers benzylic, selective cleavage 520
Ethers by reductive dimerization of aldehydes or ketones 1182
Ethers by the heteroatom Diels-alder reaction 1669
Ethers by the Williamson ether synthesis 1669
Ethers by transetherification 480 1669
Ethers cleavage of 1537
Ethers cleavage with acetic anhydride 489
Ethers cleavage with acetyl tosylate 490
Ethers cleavage with aniline salts 503
Ethers cleavage with HBr 465 520
Ethers cleavage with HI 465 519
Ethers cleavage with suifinic acids 494
Ethers cleavage with sulfuric acid 465
Ethers demethylation, with thiolate ions 496
Ethers dichloromethyl methyl, reaction with aromatic compounds 717
Ethers from acetals 528 545 1669
Ethers from alcohols 479 482 996 1669
Ethers from aldehydes 1182 1669
Ethers from alkenes 996 1669
Ethers from alkoxides 1669
Ethers from alkyl halides 477 1669
Ethers from aroxides 1669
Ethers from aryl halides 1669
Ethers from diazo compounds 479 1669
Ethers from esters 1550 1669
Ethers from ethers 480 1669
Ethers from Grignard reagents 796 1669
Ethers from inorganic esters 1669
Ethers from ketals 528 545 1669
Ethers from ketones 1182 1669
Ethers from onium ions 1669
Ethers from onium salts 482 1669
Ethers from peroxides 796
Ethers from peroxyesters 1669
Ethers from phenols 1669
Ethers from thiono esters 1550 1669
Ethers halo, elimination reactions 1344
Ethers hydrogenolysis of 528
Ethers methyl, from alcohols and diazomethane 479
Ethers oxidation with dioxirane 1525
Ethers oxidation with ruthenium tetroxide 1534
Ethers propargyl vinyl, Claisen rearrangement 1452
Ethers propargylic, reaction with Grignard reagents 546
Ethers reaction with acyl halides 483
Ethers reaction with alcohols 480
Ethers reaction with alkyl halides 492
Ethers reaction with amide bases 1328
Ethers reaction with carboxylic acids 486
Ethers reaction with Lewis acids 520
Ethers reaction with organolithium reagents 1328 1421
Ethers reaction with oxonium salts 492
Ethers symmetrical, preparation of 480
Ethers thio see “Thioethers”
Ethers, cyclic and elimination reactions 1329
Ethers, cyclic cleavage with HI 520
Ethers, cyclic from alcohols 919
Ethers, cyclic from diols 480
Ethers, cyclic from lactones 1548 1550
Ethers, cyclic from thionolactones 1215
Ethers, cyclic oxidation of 1534
Etheylenediammetetraacetic acid see “EDTA”
Ethoxide reaction with 1,1-dichloroethene 428
Ethyl acetoacetate, alkylation of 459
Ethyl chloroformate, reaction with enamines 788
Ethyl diazoacetate, and homologation of ketones 1408
Ethyl ethylthiomethyl sulfoxide, alkylation of 556
Ethyl fluoride, reaction with mesitylene and 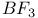 678 678
Ethyl pyroglutamate see “Pyroglutamate ethyl
Ethylaluminum dichloride, and the Prins reaction 1242
ethylene see “Ethene”
Ethylene glycol, and the Bamford — Stevens reaction 1335
Ethylene oxide and strain 180
Ethylene oxide reaction with acetyl chloride 521
Ethyllithium 240
Ethylmercuric chloride 237
Ethyne bonding 9—10
Ethyne excited state 311
Exchange H-D and electrophilic aromatic substitution 695
Exchange H-D and super acids 770
Exchange H-D in hydrocarbons 769
Exciplex, in [2+2]-cycloadditions 1082
Excitation, photochemical, types 309
Excited molecules chemical processes 317—321
Excited molecules physical processes 315
Excited states and fluorescence 313—314
Excited states and internal conversion 313
Excited states and phosphorescence 315
Excited states and photochemistry 306
Excited states fate of molecules 313—317
Excited states nomenclature 310—311
Exhaustive alkylation 500
Exo addition, in the Diels — Alder reaction 1064
exo-Deuterio-exo-norbornyl acetate 1442
Exo/endo and Baldwin’s rules 283—284
Extinction coefficient 307
Extrusion of 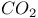 and the Story synthesis 1355 and the Story synthesis 1355
Extrusion of 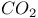 from cyclic sulfones 1354—1355 from cyclic sulfones 1354—1355
Extrusion reactions 1352
Extrusion reactions decarbonylation of ketones 1347
Extrusion reactions pyrazolines 1353
Extrusion reactions the Ramberg — Backlund reaction 1343
F strain and basicity 3436
Face strain and basicity 346
Favored reactions, and Baldwin’s rules 283—284
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -keto esters
-keto esters  -halo ketones
-halo ketones 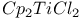
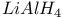
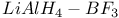
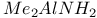
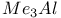
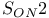 mechanism
mechanism 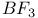
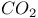 and the Story synthesis
and the Story synthesis