|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Cis/trans isomers and E2 elimination 1301 1304
Cis/trans isomers and electrophilic addition to alkenes 971—972
Cis/trans isomers and elimination 1317—1318 1333
Cis/trans isomers and fused ring compounds 161—163
Cis/trans isomers and meso compounds 161
Cis/trans isomers and monocyclic compounds 160—161
Cis/trans isomers and nomenclature 157
Cis/trans isomers and ozonolysis 1524
Cis/trans isomers and push — pull compounds 159
Cis/trans isomers and small rings 158
Cis/trans isomers and the Shapiro reaction 1335
Cis/trans, isomers strained molecules 189
Cis/trans, isomers thermal interconversion 158—159
Cisisomers 156—163
Cisoid conformation, of dienes in the Diels — Alder reaction 1064
Claisen condensation 236 552 569—570
Claisen condensation, retro 813
Claisen reaction 1224
Claisen reaction and the Reformatsky reaction 1224
Claisen rearrangement 1446 1449—1452 1454
Claisen rearrangement amine- 1452
Claisen rearrangement and accompanying Cope rearrangement 1450
Claisen rearrangement and electronic effects 1451
Claisen rearrangement and enantioselectivity 1452
Claisen rearrangement and enolene rearrangement 1450
Claisen rearrangement and Friedel — Crafts alkylation 1452
Claisen rearrangement and high pressure 457
Claisen rearrangement and homodienyl [1,5]-H sigmatropic shift 1450
Claisen rearrangement and substituent effects 1451
Claisen rearrangement and the Diels — Alder reaction 1450
Claisen rearrangement and the Willgerodt reaction 1568
Claisen rearrangement and [1,5] homosigmatropic rearrangement 1450
Claisen rearrangement Ireland — Claisen 1452
Claisen rearrangement with allyl vinyl ethers 1452
Claisen rearrangement with enolate anions 1452
Claisen rearrangement with propargyl vinyl ethers 1452
Claisen rearrangement, abnormal products 1450
Claisen rearrangement, mechanism 1449—1450
Claisen rearrangement, para- 1449
Claisen rearrangement, retro 1450
Claisen rearrangement, thio- 1452
Claisen — Schmidt reaction 1221
Clathrates 109; see “Inclusion compounds”
Clays and acetal hydrolysis 467
Clays and azide formation 515
Clays and enamine formation 1186
Clays and formation of nitriles 1195
Clays and nitration of aromatic compounds 697
Clays and reductive alkylation of amines 1188
Clays and the Friedel — Crafts reaction 708
Clays and transetherification 481
Clays Montmorillonite K-10 457
Cleavage and hydrogenation of adamantanes 815
Cleavage of alkoxides 811
Cleavage of alkyl groups from aromatic compounds 730—731
Cleavage of cyclobutanes 1081
Cleavage of Diels — Alder adducts 1069
Cleavage of diketones 812
Cleavage of ethers 489 490 1537
Cleavage of hydrocarbons, with super acids 814
Cleavage of keto esters 812
Cleavage of ketones 813
Cleavage of phosphonate esters 1331
Cleavage of sulfo groups from aryl sulfonic acids 734—735
Cleavage of tetrahydrofuran 490
Cleavage reductive, of N — allyl amines 1559
Cleavage, by Eschenmoser — Tanabe reaction 1347
Cleavage, Norrish type I 1354
Cleavage, oxidative by the Barbier — Wieland procedure 1526
Cleavage, oxidative of alkenes 1525—1526
Cleavage, oxidative of amino alcohols 1520
Cleavage, oxidative of aromatic compounds 1526
Cleavage, oxidative of diamines 1520
Cleavage, oxidative of diketones 1520
Cleavage, oxidative of diols 1519—1521
Cleavage, oxidative of epoxides 1520
Cleavage, oxidative of hydroxy aldehydes 1520
Cleavage, oxidative of hydroxy ketones 1520
Cleavage, oxidative of keto aldehydes 1520
Cleavage, oxidative with ozone 1522—1525
Cleavage, oxidative with the Lemieux — von Rudloff reagent 1526
Cleavage, photochemical 312
Clemmensen reduction 1547
Clorox, and oxidative cleavage of ketones and aldehydes 1521
CNDO method 34
Cobalt catalysts, and the Panson — Khand reaction 1090
Cobalt salen complexes, and epoxidation of alkenes 1053
Cobalt — salen catalysts, and epoxide hydrolysis 468
Collidine, and Lil in reactions with esters 522
Collins’reagent 1514
Collman’s reagent 562
Combustion, heat of 24
Combustion, heat of and elimination reactions 1315
Common rings, and strain 185
Common — ion effect 395 451
Compendium of Organic Synthetic Methods 1628
Complexes 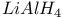 , with carbonyls 1202 , with carbonyls 1202
Complexes  , and electrophilic aromatic substitution 679 , and electrophilic aromatic substitution 679
Complexes  , for SNAr reaction of picryl chloride 852 , for SNAr reaction of picryl chloride 852
Complexes  , zirconocene, oxidation of alcohols 1515 , zirconocene, oxidation of alcohols 1515
Complexes  -allyl palladium, and enolate anions 551 -allyl palladium, and enolate anions 551
Complexes  -allyl, mechanism 772 -allyl, mechanism 772
Complexes  and electrophilic aromatic substitution 676 and electrophilic aromatic substitution 676
Complexes cobalt salen, and epoxidation of alkenes 1053
Complexes in aryl radicals 906
Complexes in the Kolbe — Schmitt reaction 718
Complexes, crown ethers 105—109
Complexes, cryptates 105—09
Complexes, cyclobntadiene — nickel 1090
Complexes, EDA, in photochemical [2+2-cycloadditions 1082
Complexes, electron donor — acceptor 102—104
Complexes, encounter, and electrophilic aromatic substitution 680
Complexes, hexacarbonyldicobalt — alkynes 1091
Complexes, Lewis acids — dienophiles 1065
Complexes, manganese porphyrin, and epoxidation of alkenes 1053
Complexes, metal carbene 1086
Complexes, nickel tetracarbonyl and allylic halides 452
Complexes, rhodium — phosphine, cyclization of dialdehydes 1564
Comprehensive Organic Functional Group Transformations 1624
Comprehensive Organic Synthesis 1624
Comprehensive Organic Transformations 1627
Computational methods and molecular mechanics 178-180
Computational methods and potential energy 179
Computational methods and small rings 181
Computational methods, ab initio 34
Computational methods, force — field calculations 178
Computers aldol 1218—1223
Computers and force — field calculations 178
Computers and molecular mechanics 178—180
Computers benzoin 1243
Computers Claisen 569—570
Computers Darzens glycidic ester 1230
Computers Dieckmann 5569—570
Computers Knoevenagel 1225—1228
Computers Stobbe 1224
Computers, ab initio calculations 34
Computers, potential energy calculations 179
Concerted Cope rearrangement 1447
Concerted reactions 1068
Concertedness, of rearrangements 1381
Condensations acyloin 1562
Conducted tour mechanism 766 771
Configuration and 1,2-shifts 1380
Configuration and ester hydrolysis 472
Configuration and Walden inversion 391
Configuration definition 167
Configuration inversion with Grignard reagents 236
Configuration, absolute see “Absolute configuration”
Conformational analysis 167—178
Conformational effects, in the E2 elimination 1305
| Conformational energy, of butane 169—170
Conformational isomer 167; see “Isomer”
Conformational stability, and optical activity 130
Conformational transmission, and strain 366
Conformations 167—178
Conformations A-value energies 174
Conformations and acid strength 347
Conformations and amides 171
Conformations and energy barrier 168
Conformations and molecular mechanism 167
Conformations and Newman projections 168
Conformations and reactivity 366
Conformations and solvent effects 170—171
Conformations and the anomeric effect 176—177
Conformations anti — periplanar, and E2 elimination 1300
Conformations anti- 169
Conformations anticlinal 169
Conformations axial, of cyclohexanones 176
Conformations boat, and the Cope rearrangement 1446
Conformations chair, and E2 elimination 1302
Conformations chair, and the Cope rearrangement 1446
Conformations cyclohexane derivatives 175
Conformations cyclohexane derivatives, stability 174
Conformations cyclopropylalkenes 182
Conformations definition 167
Conformations dioxane derivatives 175—176
Conformations dioxanes, and homoanomeric interactions 176
Conformations dithianes 176
Conformations energy and ethane 168
Conformations gauche 169
Conformations gauche and butane 169—170
Conformations Lewis acid coordination 348
Conformations of diazo ketones 1407
Conformations of rings 172—178
Conformations of rings, pseudorotation 178
Conformations open chain systems 167—172
Conformations other than six — membered 177—178
Conformations simple alkanes 168
Conformations six — membered rings containing heteroatoms 175—177
Conformations substituted cyclohexanes 173—174
Conformations syn — periplanar 169
Conformations synclinal 169
Conformations tub 57 135
Conformations vinyl carbanions 766
Conjugate addition 979—981; see “Addition conjugate”
Conjugate addition definition 976
Conjugate addition enantioselectivity 1029
Conjugate reduction see “Reduction conjugate”
Conjugated carbonyl compounds, reduction with boranes 1200
Conjugated carbonyls, reaction with Grignard reagents 1205
Conjugated carbonyls, reaction with HCN 1239
Conjugated carbonyls, reaction with organolithium reagents 1205
Conjugated circuit model for aromaticity 48
Conjugated compounds acyloxylation with organocuprates 1033
Conjugated compounds and Michael reactions 1022—1024
Conjugated compounds and the Robinson annulation 1222
Conjugated compounds by the Knoevenagel reaction 1225
Conjugated compounds by [2,3]-sigmatropic rearrangement 1454
Conjugated compounds epoxidation of 1052
Conjugated compounds from esters and aldehydes or ketones 1223
Conjugated compounds from selenoxides 1336
Conjugated compounds from the Pauson — Khand reaction 1091
Conjugated compounds, reaction with aldehydes 1212
Conjugated compounds, reaction with aldehydes and cyanide 1033
Conjugated compounds, reaction with borane 1031
Conjugated compounds, reaction with borinates 1031
Conjugated compounds, reaction with halogens 1042
Conjugated compounds, reaction with hydroperoxide anion 1053
Conjugated compounds, reduction of 1007
Conjugated ketones see “Ketones”
Conjugation and carbanions 229—230
Conjugation and double bond migration 771
Conjugation and photochemistry 310
Conjugation and rate of nucleophilic addition 1174
Conjugation cross- 39—40
Conjugation, double or triple bonds 36—37
Conrotatory rotation, in electrocyclic rearrangements 1427
Contact ion pairs 398
Cope elimination 1324 1333 1421
Cope reaction 1324 1333
Cope reaction, accompanying Claisen rearrangement 1450
Cope rearrangement 1444—1449
Cope rearrangement and bullvalene 1448
Cope rearrangement and fluxional structures 1447
Cope rearrangement and high pressure 457
Cope rearrangement and valence tautomerism 1447
Cope rearrangement aza- 1445
Cope rearrangement azo- 1452
Cope rearrangement catalyst for 1446
Cope rearrangement of 1,5-diynes 1445
Cope rearrangement, degenerate 1447
Cope rearrangement, diradical mechanism 1447
Cope rearrangement, nonconcerted 1447
Cope rearrangement, oxy- 1445 1452
Cope rearrangement, transition state 1446
Copper chromite, and dehydrogenation of alcohols 1515
Copper salts, and the Ullmann ether synthesis 863
Copper, reaction with aryl carboxylic acids 732
Corannulene 71
Corey — Winter reaction 1340
Corey’s reagent, and oxidation alcohols 1515
Counterions ambident nucleophiles 460
Counterions and nucleophilic reactivity 460
Counterions, definition 219
Coupling and carbon nucleophiles 534
Coupling and the Heck reaction 930
Coupling and the Wurtz reaction 535
Coupling of acyl halides 568
Coupling of alcohols 544
Coupling of alkanes 926
Coupling of alkynes 561
Coupling of aryl diazonium salts 937
Coupling of aryl halides 870
Coupling of boranes 939
Coupling of diazonium salts 700
Coupling of Grignard reagents 938
Coupling of hydrocarbons 786
Coupling of organocuprates 939
Coupling of organocuprates and sulfonate esters 543
Coupling of organonietallics and alkyl halides 536 540—541
Coupling of propargyl halides 543
Coupling of thiohydroxamic esters 942
Coupling reactions, organocuprates and alkyl halides 538—540
Coupling with aryl halides 868
Coupling, reductive, of aldehydes and ketones 1559—1561
Covalent character, and organometallics 234
Cram’s rule 1172
Cram’s rule and asymmetric reduction of ketones 1201
Cram’s rule and synthesis 147—148
CRC Handbooks 1617 1619
Criegee mechanism, for ozonolysis 1523—1525
Cross — conjugation 39—40
Cross — coupling, and carbon nucleophiles 534
Crossed Cannizzaro reaction 1564
CrossFire 1616
Crossover experiments 393 1378
Crossover experiments and Fries rearrangement 727
Crowding, steric strain 189
Crown ethers and amide formation 514
Crown ethers and chirality 136
Crown ethers and formation of alkyl hydroperoxides 492
Crown ethers and ketone enolates with esters 571
Crown ethers and oxidative cleavage with 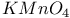 1526 1526
Crown ethers and resolution 151 152
Crown ethers as phase transfer catalysts 455
Crown ethers azo, isomerization 320
Crown ethers complexation 105—109
Crown ethers, definition 105
Crown ethers, host — guest 105
Crown ethers, metal ionic binding 105
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



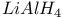 , with carbonyls
, with carbonyls  , and electrophilic aromatic substitution
, and electrophilic aromatic substitution  and electrophilic aromatic substitution
and electrophilic aromatic substitution