|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Smith M.B., March J. — March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure |
|
|
 |
| Предметный указатель |
Crown ethers, reaction with amines and 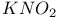 494 494
Cryptands and nucleophilic substitution 443
Cryptands and resolution 152
Cryptands as phase transfer catalysts 455
Cryptands, calixaromatic compounds 106
Cryptands, conformational isomers 106
Cryptands, cryptophanes 106—107
Cryptands, definition 106
Cryptands, hemispherands 106—107
Cryptands, lariat ethers 107
Cryptands, metal ion binding 106
Cryptands, molecular recognition 108
Cryptands, podands 106—107
Cryptands, spherands 106
Cryptands, starands 107
Cryptates see “Cryptands”
Cryptates and out — in isomers 163
Cryptates, complexation 105—109
Cryptates, definition 106
Cryptophanes 106—107
Crystallization, and resolution 153
Crystallographic scale, of acid strength 328
Cubane 182—183 1459
Cubene 191
Cubyl cation, ab initio calculations and the  reaction 397 reaction 397
Cubylcarbinyl radial 1390
Cumene, from benzene 709
Cumulenes from alkyne epoxides 1344
Cumulenes from alkynes 1344
Cumulenes from dihalides 1343
Cumulenes, cyclic 187
Cuneane 1459
Cuprates see “Organocuprates”
Cupric acetate, reaction with carboxylic acids and lead tetraacetate 1528
Cupric chloride, and Meerwem arylation 929—930
Cuprous chloride and reaction of hydroperoxides and hydrocarbons 922
Cuprous chloride, reaction with aryl diazonium salts 935
Cuprous cyanide and isocyanide formation 562
Cuprous cyanide, reaction with acyl halides 573
Cuprous cyanide, reaction with aryl diazonium salts 936
Cuprous cyanide, reaction with aryl halides 867
Cuprous cyanide, reaction with arylthallium compounds 802
Curtius rearrangement 1380 1384 1406 1412
Cyanamide, reaction with alkyl halides 501
Cyanamide, reaction with ammonia 1192
Cyanamides by the von Braun reaction 1663
Cyanamides from amines 522
Cyanamides from cleavage of tertiary amines 1663
Cyanamides from dehydration of disubstituted ureas 1663
Cyanates, from reaction of aroxides and cyanogen halides 1663
Cyanation, of ketones 786
Cyanation, of nitro compounds 786
Cyanides see “Nitriles”
Cyanides and phase transfer reactions 561
Cyanides and the Kiliani — Fischer synthesis 1240
Cyanides and the Strecker synthesis 1240
Cyanides as an ambident nucleophile 459
Cyanides in HMPA, cleavage of methyl esters 562
Cyanides, acyl see “Acyl cyanides”
Cyanides, alkyl, from trialkylcyanoborates 802
Cyanides, ambident nucleophiles 459
Cyanides, potassium reaction with hydrazones 1241
Cyanides, potassium, and phase transfer catalysts 456
Cyanides, potassium, reaction with aldehydes 1243
Cyanides, reaction with alkyl halides 561—562
Cyanides, reaction with aromatic nitro compounds 876
Cyanides, reaction with bisulfite addition products 1240
Cyanides, silver, and alkylation 461
Cyanides, sodium reaction with boranes 1423
Cyanides, sodium, and alkylation 461
Cyanides, tosyl, reaction with enolate anions 786
Cyanides, vinyl, from vinyl bromides 562
Cyanides, vinyl, from vinyl copper reagents 802
Cyano amines, from cyanohydrins 502
Cyano ketones 786
Cyano ketones and ring expansion 813
Cyano ketones cleavage of 813
Cyanoacetic ester synthesis 549
Cyanoamines by the Mannich reaction 1663
Cyanoamines by the Strecker synthesis 1663
Cyanoamines from aldehydes 1663
Cyanoamines from amines 1663
Cyanoamines from aromatic amines 1663
Cyanoamines from cyanohydrins 1663
Cyanoamines from ketones 1663
Cyanoamines from nitriles 1663
Cyanoamines fromdehydes 1663
Cyanoamines fromimines 1663
Cyanoborohydride see “Sodium cyanoborohydride”
Cyanoborohydride, sodium, and reduction of tosylhydrazones 1548
Cyanoborohydride, sodium, reduction of oximes 1203
Cyanoethylation 976
Cyanogen bromide, reaction with amines 522
Cyanogen bromide, reaction with carboxylate salts 1246
Cyanogen chloride, reaction with alcohols 1183
Cyanogen chloride, reaction with carboxylate salts 1246
Cyanohydrins, alkylation of 553
Cyanohydrins, from aldehydes or ketones and HCN 1239
Cyanohydrins, hydrolysis of 1179
Cyanohydrins, reaction with ammonia 502
Cyclacenes 67
Cyclic alcohols, from di magnesium compounds and esters 1214
Cyclic amines see “Amines cyclic”
Cyclic amines, cleavage with cyanogen bromide 523
Cyclic anhydrides from diacids 491
Cyclic anhydrides, reaction with ammonia 508
Cyclic compounds also see “Rings”
Cyclic compounds and cis/trans isomers 160—161
Cyclic compounds and E2 elimination 1304
Cyclic compounds by Dieckmann cyclization 1646
Cyclic compounds by Favorskii rearrangement 1647
Cyclic compounds by Pschorr arylation 1646
Cyclic compounds by the acyloin condensation 1647
Cyclic compounds by the Diels — Alder reaction 1646
Cyclic compounds by the Story reaction 1647
Cyclic compounds by the Thorpe — Ziegler reaction 1647
Cyclic compounds by the Wnrtz reaction 1646
Cyclic compounds by Wolff rearrangement 1647
Cyclic compounds c and r nomenclature 161
Cyclic compounds from  -bond rearrangements 1647 -bond rearrangements 1647
Cyclic compounds from 1,3-diols 1646
Cyclic compounds from 1,4-dihalides 1646
Cyclic compounds from aldehydes 1646
Cyclic compounds from alkenes 1646
Cyclic compounds from alkynes 1646
Cyclic compounds from all-carbon [3+2]-cycloadditions 1646
Cyclic compounds from aromatic rings 1646
Cyclic compounds from carbenes 1646
Cyclic compounds from carbenoids 1646
Cyclic compounds from carboxylic esters 1646
Cyclic compounds from conjugated dienes 1647
Cyclic compounds from contraction of rings 1647
Cyclic compounds from cyclic boranes 1647
Cyclic compounds from cyclic ketones 1647
Cyclic compounds from cyclic peroxides 1647
Cyclic compounds from cycloaddition reactions 1646
Cyclic compounds from di magnesium compounds 1646
Cyclic compounds from dialdehydes 1647
Cyclic compounds from diallylic halides 1646
Cyclic compounds from dicarboxylic acids 1646
Cyclic compounds from dienes 1646
Cyclic compounds from diesters 1647
Cyclic compounds from dihalobiphenyls 1646
Cyclic compounds from diketones 1647
Cyclic compounds from dimagnesio compounds 1646
Cyclic compounds from dinitriles 1647
Cyclic compounds from diynes 1646 1647
Cyclic compounds from expansion of rings 1647
Cyclic compounds from extrusion of CO from cyclic ketones 1647
Cyclic compounds from extrusion of N2from pyrazoles 1647
| Cyclic compounds from extrusion of N2from pyrazolines 1647
Cyclic compounds from extrusion of SO2from cyclic sulfones 1647
Cyclic compounds from halo alkenes 1646
Cyclic compounds from halo carbonyl compounds 1646
Cyclic compounds from halo ketones 1647
Cyclic compounds from internal aldol reactions 1646
Cyclic compounds from internal condensation of diesters 1646
Cyclic compounds from internal coupling 1646
Cyclic compounds from internal malonic ester synthesis 1646
Cyclic compounds from internal Mukaiyama aldol reactions 1646
Cyclic compounds from internal Wittig reactions 1646
Cyclic compounds from intramolecular arylation 1646
Cyclic compounds from intramolecular Friedel — crafts acylation 1646
Cyclic compounds from intramolecular Friedel — crafts alkylation 1646
Cyclic compounds from intramolecular insertion of carbenes 1646
Cyclic compounds from intramolecular insertion of carbocations 1646
Cyclic compounds from keto esters 1647
Cyclic compounds from ketones 1646
Cyclic compounds from metathesis of dienes 1647
Cyclic compounds from oxidative cyclization 1647
Cyclic compounds from radical cyclization 1646
Cyclic compounds from ring contraction 1647
Cyclic compounds from Scholl ring closure 1646
Cyclic compounds from the di — rc-methane rearrangement 1647
Cyclic compounds from unsaturated aldehydes 1646
Cyclic compounds from unsaturated Grignard reagents 1646
Cyclic compounds from vinylcycloalkanes 1647
Cyclic compounds from vinylcycloalkenes 1647
Cyclic compounds from Wagner — Meerwein rearrangements 1647
Cyclic compounds from [1j] sigmatropic migrations of carbon 1647
Cyclic compounds, small rings and strain 180—184
Cyclic ketones by the Ruzicka cyclization 574
Cyclic ketones from dicarboxylic acids 574
Cyclic ketones from keto esters 1561
Cyclic ketones, oxidative cleavage 1521
Cyclic ketones, oxidative cleavage with NaOCl 1522
Cyclic ketones, reaction with hydrazoic acid 1414
Cyclic ketones, ring expansion with diazomethane 1408
Cyclic substrates, effect on nucleophilic substitution 437
Cyclic sulfates, ring opening 462
Cyclization also see “Ring closing”
Cyclization Bergman 1432
Cyclization by acyloin condensation 1562
Cyclization by alkene metathesis 1458
Cyclization by Fischer indole synthesis 1452
Cyclization by McMurry reaction 1561
Cyclization by photocyclization of stilbenes 1436
Cyclization by the aldol reaction 1222
Cyclization by the Hofmann — Laffler reaction 1462
Cyclization by the Thorpe — Ziegler reaction 1239
Cyclization by the Wittig reaction 1236
Cyclization cationic, polyene 1019
Cyclization Nazarov 1021
Cyclization of alkene — organolithium reagents 1025
Cyclization of bis — allylic halides 542
Cyclization of dialdehydes with 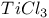 1560 1560
Cyclization of esters and dimagnesium compounds 1214
Cyclization of keto esters 1561
Cyclization, radical 403 985 1039—1041
Cyclization, radical and Baldwin’s rales 978 986
Cyclization, radical and [1,7]-H sigmatropic rearrangements 1440
Cyclization, radical of dialdehydes 1561
Cyclization, radical to C=N and C=O 1244
Cyclization, radical to conjugated compounds 1032
Cyclization, radical with dienes 978
Cyclization, radical with polyenes 1020
Cyclization, radical [2+2+2]- 1093
Cycloadditions [1,2]-of carbenes 1084
Cycloamyloses see “cyclodextrins
Cyclobutadienes and antiaromaticity 58—60
Cyclobutadienes and aromaticity 57
Cyclobutadienes and the Diels — Alder reactions 59
Cyclobutadienes derivatives 59—60
Cyclobutadienes from alkyne dimerization 1090
Cyclobutadienes, matrix trapping 59
Cyclobutadienes, metal complexes 60
Cyclobutadienes, nickel complex 1091
Cyclobutadienes, NMR of derivatives 60
Cyclobutadienes, trapping in carcerands 59
Cyclobutane and angle strain 182
Cyclobutane, conformation 177
Cyclobutanes cycloreversion 1081
Cyclobutanes divinyl, Cope rearrangement 1445
Cyclobutanes from alkenes 1077—1084
Cyclobutanes from cyclic sulfones 1354—1355
Cyclobutanes thermal cleavage 1081
Cyclobutanes vinyl, rearrangement 1444
Cyclobutanones from ketenes and alkenes 1077
Cyclobutanones photochemical decarbonylation 1354
Cyclobutene, from cyclopropylmethyl carbine 1400
Cyclobutenes HOMO 1428
Cyclobutenes, photochemical ring opening 1428
Cyclobutenes, thermal ring opening 1428
Cyclobutenes, thermolysis 1426
Cyclobutyl dications, and aromaticity 58
Cyclobutylcarbinyl see “Cyclobutylmethyl”
Cyclobutylmethyl cations, rearrangement 418
Cyclobutylmethyl, neighboring group effects 417
Cyclodecapentanene, and aromaticity 57
Cyclodextrins and channel — type complexes 113
Cyclodextrins and chlorination of anisole 686
Cyclodextrins and hydrogen bonding 111—112
Cyclodextrins and inclusion compounds 111—113
Cyclodextrins and resolntion 152
Cycloheptatriene 52—53 1087
Cycloheptatrienes, from norcaradienes 1427
Cycloheptatrienyl anion 52—53
Cycloheptatrienyl anion and antiaromaticity 62
Cycloheptatrienyl cation see “Tropylium ion”
Cycloheptyne 187
Cyclohexacenes 67
Cyclohexadienes by Cope rearrangement 1445
Cyclohexadienes thermal cleavage 1430
Cyclohexadienes thermolysis 1426
Cyclohexadienone 75
Cyclohexadienone reaction with acid 1402
Cyclohexadienones 2.5-, photolysis of 1461
Cyclohexane conformational energies 172
Cyclohexane conformations 172—175
Cyclohexane derivatives and conformations 175
Cyclohexane oxidation to cyclohexanone 1532
Cyclohexane substituted, and conformations 173—174
Cyclohexane substituted, and optical activity 175
Cyclohexane substituted, and stereoisomers 175
Cyclohexanone derivatives, % axial 176
Cyclohexanone from cyclohexane 1532
Cyclohexenes, by the Diels — Alder reaction 1062
Cyclohexyne 187
Cyclononyne 187
Cyclooctadiene, from cyclooctene oxide 1341
Cyclooctatetraene 1434
Cyclooctatetraene and antiaromaticity 62
Cyclooctatetraene and aromaticity 57
Cyclooctatetraene from acetylene 1089
Cyclooctatetraene tub conformation 57 135
Cyclooctatriene 1433
Cyclooctene and helical chirality 135
Cyclooctene and trans double bonds 158
Cyclooctene photochemical isomerization 320
Cyclooctyne 187
Cyclopentadiene anion and aromaticity 52
Cyclopentadiene anion and reaction with alkyl halides 550
Cyclopentadiene reaction with ketene 1080
Cyclopentadienone Diels Alder reactions 1348
Cyclopentadienone stability of 53
Cyclopentadienyl anion 231
Cyclopentadienyl anion and aromaticity 52
Cyclopentadienyl anion, reaction with alkyl halides 550
Cyclopentadienyl carbanions 230
Cyclopentadienyl cation, and antiaromaticity 60—61
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



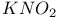
 reaction
reaction  -bond rearrangements
-bond rearrangements