|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Patai S. — The Chemistry of Organic Silicon Compounds (part 1-2) |
|
|
 |
| Предметный указатель |
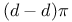 bonding 273 1433 1435 1436 1442 bonding 273 1433 1435 1436 1442
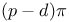 bonding 896—897 901 bonding 896—897 901
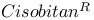 1154 1187 1154 1187
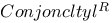 1153 1153
 -conjugation 4 -conjugation 4
 complexes 273—274 1435 complexes 273—274 1435
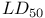 values 1151 1183 1184 values 1151 1183 1184
 -Hydrogen, activation of 954—955 -Hydrogen, activation of 954—955
 -Hydroxy ketones, synthesis of 771 798 803 -Hydroxy ketones, synthesis of 771 798 803
 -Keto esters asymmetric reduction of 1513—1514 -Keto esters asymmetric reduction of 1513—1514
 -Keto esters synthesis of 787 -Keto esters synthesis of 787
 -Ketoacylamino esters, asymmetric reduction of 1514 -Ketoacylamino esters, asymmetric reduction of 1514
 -Methylstyrene, hydrosilylation of 1486—1487 -Methylstyrene, hydrosilylation of 1486—1487
 -Siloxyaidehydes, synthesis of 1468 -Siloxyaidehydes, synthesis of 1468
 -Silyl enol 196 -Silyl enol 196
 -Silyl ketones 194 196 -Silyl ketones 194 196
 -Silyl organometallic reagents 787—789 -Silyl organometallic reagents 787—789
 -Silyl substitution, -Silyl substitution,  reactivity affected by 203 reactivity affected by 203
 -Silylallyl anions, in Peterson reaction 949 -Silylallyl anions, in Peterson reaction 949
 -Silylaziridines 954 -Silylaziridines 954
 -Silylcarbanions 450 495 496 907—908 942—951 -Silylcarbanions 450 495 496 907—908 942—951
 -Silylcarbanions formation of 942—944 -Silylcarbanions formation of 942—944
 -Silylcarbanions in Peterson reaction 944—951 -Silylcarbanions in Peterson reaction 944—951
 -Silylcarbenium ions 193—196 -Silylcarbenium ions 193—196
 -Silylcarbonium ions 904—905 920 929 -Silylcarbonium ions 904—905 920 929
 -Silylcarboxylic acids, acidity of 816 -Silylcarboxylic acids, acidity of 816
 -Silylepoxides 798 951—954 -Silylepoxides 798 951—954
 -Silylvinyl alcohols 80 -Silylvinyl alcohols 80
 -Silylvinyl carbanions, in Peterson reaction 950 -Silylvinyl carbanions, in Peterson reaction 950
 -Silylvinyl cation 194 -Silylvinyl cation 194
 -stabilization energies 386 -stabilization energies 386
 -Epoxysilanes rearrangement of 952 -Epoxysilanes rearrangement of 952
 -Epoxysilanes ring-opening reactions of 953 -Epoxysilanes ring-opening reactions of 953
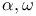 -Bis(trialkylammoniomethyl)oligodimethylsiloxanes 1155 -Bis(trialkylammoniomethyl)oligodimethylsiloxanes 1155
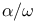 -substituents, interactions of 469—470 -substituents, interactions of 469—470
 -Azido alcohols 795 -Azido alcohols 795
 -Carbonium stabilization 920 -Carbonium stabilization 920
 -Cyclocitral, sila-analogue of 1192 -Cyclocitral, sila-analogue of 1192
 -Hydroxypyrazoles, synthesis of 791 -Hydroxypyrazoles, synthesis of 791
 -Hydroxysilane, synthesis of 789 -Hydroxysilane, synthesis of 789
 -Iodo acetals, synthesis of 766 -Iodo acetals, synthesis of 766
 -Iodo ketones, synthesis of 766 -Iodo ketones, synthesis of 766
 -Iodoenones, synthesis of 766 -Iodoenones, synthesis of 766
 -Ionone, sila-analogue of 1192 -Ionone, sila-analogue of 1192
 -Pinene, hydrosilylation of 673 -Pinene, hydrosilylation of 673
 -Silyl carbonyl compounds, synthesis of 954 -Silyl carbonyl compounds, synthesis of 954
 -Silyl elimination reactions 1448 -Silyl elimination reactions 1448
 -Silylcarbenium ions 196—198 -Silylcarbenium ions 196—198
 -Silylcarbonium ions 905—906 917 929 930 938 -Silylcarbonium ions 905—906 917 929 930 938
 -Silylcarbonium ions reactions controlled by formation of 940—941 -Silylcarbonium ions reactions controlled by formation of 940—941
 -Silylenolates, stereoselectivity with 936—937 -Silylenolates, stereoselectivity with 936—937
 -stabilization energies 386 -stabilization energies 386
 -Silylcarbenium ions 198 -Silylcarbenium ions 198
 -Silylcarbonium ions 906—907 -Silylcarbonium ions 906—907
 -Silylcarbonium ions reactions involving 941—942 -Silylcarbonium ions reactions involving 941—942
 -bond energies 8 12 387 -bond energies 8 12 387
 -bonded species, enthalpies of formation of 387 -bonded species, enthalpies of formation of 387
 -bonded species, PE spectra of 619—628 -bonded species, PE spectra of 619—628
 -complexes 294 -complexes 294
 -complexes NMR spectra of 541—542 -complexes NMR spectra of 541—542
 -inductive effect 896 -inductive effect 896
 -polarization 1009 1011 1012 -polarization 1009 1011 1012
(-)Menthone, hydrosilylation of 320 321
(-)Menthoxy derivatives, optically active silanes synthesized from 307—310
(-)Menthyl derivatives, asymmetric reduction of 1513
(-)Methyl-naphth-l-phenylsilanol, synthesis of 723
(2-Chloroethyl)bis(phenylmethoxy)methylsilane, biological activity of 1157
(2-Triorganylsilylethylidene)phosphoranes, allylsilanes synthesized using 672
(N-Methyltrifluoroacetamido)trimethyl-silane 710
(Trimethylsilyl)ethynyl cations, NMR spectra of 519
(Trimethylsilyl)methyl anion 494
(Trimethylsilyl)trimethyldisilane, reactions of 1024 1040
1, 2-Acetoxy migration 967
1,1-Dimethyl-1-silacyclopentenes 672 694 696
1,1-Diphenylsila-2-cyclohexanone 986
1,1-Diphenylsilacyclobutane 972
1,1-Diphenylsilene 973
1,2,4,5-Tetr -f-butyl-1,2,4,5-tetraphospha-3-silaspiro[2.2]pentane 1381 -f-butyl-1,2,4,5-tetraphospha-3-silaspiro[2.2]pentane 1381
1,2,4-Triphosphabuta-1, 3-dienes, silylsubstituted 1373—1374
1,2-Bis(trimethylsilyl)ethane 617
1,2-Dioxetanes, synthesis of 802
1,2-Diphosphatrisilacyclopentane derivatives 1382
1,2-Disilaoxetanes 696
1,2-Disiloxetanes 1038
1,2-elimination of 41
1,2-Silaoxetane 100 119
1,2-Siloxetanes, formation during photolysis 995
1,3,4,6-Tetraphosph -1, 5-dienes, silylsubstituted 1374 -1, 5-dienes, silylsubstituted 1374
1,3,7-Octatriene, hydrosilylation of 1495
1,3-Butadiynes, hydrosilylation of 1499
1,3-Cyclohexadiene, hydrosilylation of 1496
1,3-Diols, synthesis of 774
1,3-Dioxa-2,4-disilacyclobutane 97
1,3-Diphospha-2, 4-disilacyclobutanes 97 262
1,3-Diphospha-2-silacyclopentane derivatives 1382
1,3-Diphosphacyclobutenes, silyl-substituted 1373
1,3-Disilacyclobutanes 97 990
1,3-Pentadiene, hydrosilylation of 1495 1497 1499
1,3-Silyl shift 994
1-Acetoxyalkylsilanes 1197
1-Arylsilatranes, toxicity of 1151—1152
1-Chloro-1-silabicyclo[2, 2, 2]octane, synthesis of 705
1-Chlorosilatrane 829
1-Haloadamantanes, cyanation of 772
1-Heteroarylvinyltrimethylsilane 665
1-Hydroxyalkylsilanes, biotransformation of 1197
1-Methylsilyne 128
1-Naphthylphenylhalosilanes menthanolysis of 314
1-Naphthylphenylhalosilanes racemization of 870
1-Naphthylphenylmethylsilyl derivatives, nucleophilic substitution in 842 845
1-Oxa-2,7-disilacyclohept-4-ene 704—705
1-Phenyl-1-trimethylsilyl-2-ethoxymethyl-1-silacyclopropane 694
1-Silaacenaphthenes 698
1-Silacyclopropylidime 128
1-Silylsilene 127
1-Trimethylsiiyladamantane 660
1-Trimethylsilyl-oct-1-yne-3-ol 679
1-Trimethylsilylalkynes, catalytic hydrogenation of 669
1-Trimethylsilylcyclohexa-1, 4-diene 670
19-Nortestosterone, silyl derivatives of 1147
2,2-DimethyI-2,3-dihydro-2-silaphenalene 699
2,6-cis-Diphenylhexamethylcyclotetrasiloxane 1154 1187
2-(l-Naphthyl)-2-sila-1,2,3,4-tetrahydronaphthalene, resolution of 308
2-Bromo-trimethylsilyl-pyridines 692
2-Naphth-1yl-2-organyl-2-silatetralin derivatives 700
2-Phenylheptamethyltrisilane, photolysis of 1021
2-Phosph -1, 3-disilacyclopentane derivatives 1383 -1, 3-disilacyclopentane derivatives 1383
2-Sila-1,3-dithiacyclopentane 1406
2-Silaacetate anion 496
2-Silaoxetanes adducts of 1092
2-Silaoxetanes formation of 1090—1091
2-Siloxysilenes, addition reactions of 1098
2-Trimethylsilyl-4-f-butylpyridine 692
2-Trimethylsilyl-ethynyl-1,3-dioxane 678
21-Hydroxyprogesterone, synthesis of 781
3,3-Dichloro-3H-3-benzosilepin 566
3,3-Dimethyl-3-silabicyclo[3.2.0]heptane 705
3,4-Bis(trimethylsilyl)-5-ethoxycarbonylpyrazole 686
3,4-Bis(trimethylsilyl)pyrazole 686
3,4-Bis(trimethylsilyl)pyrazolium nitrate 687
3,5,7-Tris(trimethylsilyl)tricyclo[2.2.1.0]heptaphosphane 1378
3-(Trimethylsilyl)cyclohexyl brosylate, solvolysis of 941
3-Trimethylsilyl-2-propynoate 678
3-Trimethylsilyl-4, 5-dihydro-5-phenylisoxazole 691
3-Trimethylsilyl-4-phenylpyrazole 686
3-Trimethylsilyl-5-ethoxycarbonylpyrazole 686
3-Trimethylsilyl-cyclooctene 676
3-Trimethylsilyl-methylcyclopentadiene 674
3-Trimethylsilyl-methylpyrazole 688
3-Trimethylsilyl-propynal 678
| 3-Trimethylsilylindoles 800
3-Trimethylsilylpyrazoles 686—689
3-Trimethylsilylthiophene 685—686
4,5-Diaminouracil, silylation of 715
4,7,11-Tris(trimethylsilyl)pentacyclo[6.3.0.0.0.0]undecaphosphane 1378
4-Aminopyrazoles, synthesis using trimethylsilyl cyanide 771
4-Bromo-3-trimethylsilyl-pyrazole 687
4-Cyanobutenolides, synthesis of 770
5,6-Bis(trifluoromethyl)-2-silabicyclo[2, 2, 2]octa-5,7-diene 564
5-Trimethylsilyl-methylisoxazole 691
7-Silanorbornadienyl iron derivative 1432
7-Silanorbornane 699
8-Trimethylsilyl-4H-1,2,3,4-tetrakis(methylcarbony|)quinolizine 694
9, 10-Disilaphenanthrene derivatives 703
9,10-Disilaanthracene derivatives 702
9,9-Dimethyl-1,2,5,6-tetraphospha-9-silatricyclo[3.3.1.0]nonane 1381
9-Phenyl-9-silaxanthene 699
9-Silaanthracene derivatives 699
Aalbersberg, W.G.L. 915 (123) 959
Aamodt, L.C. 5 (31) 50
Ab initio calculations 62—65
Ab initio calculations carbanion stabilization 908
Ab initio calculations gas-phase acidities 814
Ab initio calculations nucleophilic substitution 857
Ab initio calculations proton affinities 821 822
Abboud, J.L.M. 810 (7) 835
Abdejaken, F. 1045 (116) 1136
Abdesaken, F. 38 (203) 54 60 108 116 120 122 167) 213 219 228 236 237 61) 294 295 314 40) 366 519 520 532 32) 533 548 549 551 988 1004 1044 1045 106) 1068 1072 1078 105) 1085 1086 1100 106) 1101 1135
Abe, R. 775 (53) 805
Abe, T. 1396 (5) 1411
Abe, Y. 424 (380) 442
Abel, E. 1218
Abel, E.W. 671 (82) 745 746 753 760 819 822 836 1364 1384 1388 1389 110) 1390 1393 1395 1396 3) 1397 10 13 14) 1400 13 22 24) 1402 1406 1410 1411
Aberg, B. 1154 (89) 1201
Abkowitz, M. 1236 (130) 1240
Aboujaoude, E.EM. 788 (107) 806
Abraham, M. 810 (7) 835
Abraham, W.R. 1144 1193 1199
Abramova, E.A. 726 (305) 758
Abrosimova, A.T. 1155 1181 1201
Acenaphthylenylsilanes 684—685
Acetamidosilane 710
Acetoxy groups, analysis of 409 420
Acetylene, hydrosilylation of 1482 1489
Acetylenes, trimethylsilyl cyanide added to 773
Acetylenic silanes 785—787 (see also «Ethynylsilanes»)
Acetylsilanes 1194—1196
Achiba, Y. 556 557 558 561 562 568 576 583 584 586 587 588 593 594 596 597 600 602 604 606 607 608 615 619 620 621 627 629 644
Achiwa, K. 791 (121) 794 795 807 1509 199b) 1525
Acidity 812—817
Acidity definitions 810
Acidity definitions, measurement of 810—812
Ackerman, J.L. 544 (127) 553
Ackermann, J. 1170 (186 188 189) 1203
Ackermann, K. 291 (415 417 418) 292 (417) 303 327 328 367 540 541 553 1438 1439 1441 1474
Activating effects 893—956
Acyclic compounds, photochemistry of 966—968 973—975 976—977
Acyclic oligosilanes, synthesis of 1210—1211
Acyclic silanes photolysis of 966—968
Acyclic silanes stereochemistry compared with cyclic silanes 352
Acyl chlorides, reactions with phosphinosilanes 1371
Acyl nitrites, trimethylsilylation of 769
Acyldisilanes, photolysis of 987
Acyloxyorganylsilanes 730—732
Acylpolysilanes 987—989
Acylsilanes NMR spectra of 519 520
Acylsilanes photolysis of 984—987
Acylsilanes synthesis of 1444 1456 1466
Acylsulfenyl halides 804
Acylthioorganylsilanes 749—750
Aczel, T. 501 (175a) 510
Adamantane derivatives 1-halo-derivative 772
Adamantane derivatives silylenium ion intermediate 1013
Adamantane derivatives trimethylsilyl derivative 660
Adams, B.R. 97 98 100 218
Adams, K.P. 1486 (53c) 1522
Adams, R.E. 414 415 418 438
Adcock, W. 660 (31) 752 902 44) 957
Additivity rules 372—374 381
Adley, A.D. 1257 1267 1287
Adoni, E. 638 (234) 652
Agakhanova, T.B. 1500 (113) 1523
Agarunov, M.JM. 372 379 380 390
Ager, D.J. 203 (355a) 224 663 752 787 806 945 946 962
Aggarwal, R.C. 210 211 225 1257 1287
Aglinlov, N.K. 425 (387) 442
Agripat, S.A. 716 (250) 757
Aguir, A.M. 1509 (200a) 1525
Ahlers, P.E. 394 (7) 434
Ahlfors, C. 1159 1185 1202
Ahlrichs, R. 63 (30b) 106 107 109 111 112 113 116 201 205 207 364) 214 218 224 225 280 303 451 506 559 (49) 646
Ahmed, W. 851 (42) 889 1279 1288
Ainshtein, A.A. 426 (403) 442
Ainstein, S.A. 431 (451) 443
Ainsworth, C. 736 (364) 737 759
Airey, W. 242 (73 74) 254 255 263 74) 295 298 549 553
Aitken, C. 48 (279) 56 1426 1440 1462 294 297) 1472 1476
Aitken, C.T. 1225 (90) 1239 1426 1435 1438 1440 1462 1472
Aiube, Z.H. 886 887 892 1124 (381) 1141
Aizpurua, J.M. 804 (165) 808
Ajimura, N. 785 (97) 806
Ajinomoto Co.Inc. 735 (360) 759
Akaba, R. 548 (146) 553
Akai, S. 785 (97) 806
Akhmedov, I.M. 1481 (6) 1493 1520 1522
Akhrem, I. 1279 (171) 1288
Akhrem, I.S. 457 482 507
Akiba, K.Y. 933 (191) 960
Akita, M. 1269 (135 136) 1287 1450 (228) 1475
Akkerman, O.S. 458 (65) 507
Akkok, S. 475 480 508
Aksamentova, T.N. 827 (107) 837 1244 1285
Aktaiay, Y. 634 (224) 652
Al-Derzi, A. 11 (58) 51 150 220 1131 1142
Al-Khayat, T.H. 915 (125) 959
Al-Shali, S.A.I. 880 (145) 884 886 145) 892
Al-Wassil, A.I. 882 (123 127) 891
Alanes, optically active organosilanes reduced by 350—351
Albanesi, T.E. 1068 1069 1086 1139
Albanov, A.I. 1256 1257 1261 120) 1286
Albaugh-Robertson, P. 932 (188) 960
Albitskaya, V.M. 1482 (33 34) 1521
Albrecht, H. 711 (227) 756
Albright, M.J. 270 (323 328) 301
Albright, T.A. 1434 (127) 1472
Alcock, N.W. 834 (145 146) 838
Alcohols, silyl-substituted 193
Aldehydes hydrosilylation of 1499—1515
Aldehydes trimethylsilyl cyanide added to 769
Aldoshin, S.M. 1254 (88) 1286
Aldous, G.L. 660 (31) 752 902 957
Aldrich, J.H. 1157 (123) 1202
Aleksandrova, Yu.A. 1323 1324 1359
Alekseev, N.V. 4 (4) 49 60 205 213 230 279 289 292 294 1249 1286
Alekseeva, G.M. 1482 1483 1521
Alekseeva, Z.I. 425 (396) 442
Alexakis, A. 765 (7) 805
Alexander, A.G. 966 (9) 1001
Alexander, G. 429 (431 432) 443 1331 1332 1360
Alexander, J.J. 1453 (246) 1475
Alexeev, N.V. 262 (279) 263 300
Alfrey, T. 1311 (125 128) 1359
Alfthan, O. 1154 1184 1201
Aliphatic compounds, electrophilic reactions in 910—942
Alkenes hydrosilylation of 1480—1487 1488—1490
Alkenes reductive silylation of 671
Alkenylpentafluorosilicates 1269—1270
Alkenylthioorganylsilanes 749
Alkenyltrifluorosilanes 1269
Alkoxides, coupling reactions with 345
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 bonding
bonding 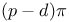 bonding
bonding 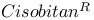
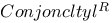
 -conjugation
-conjugation  complexes
complexes 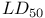 values
values  -Hydrogen, activation of
-Hydrogen, activation of  reactivity affected by
reactivity affected by  -Epoxysilanes rearrangement of
-Epoxysilanes rearrangement of 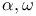 -Bis(trialkylammoniomethyl)oligodimethylsiloxanes
-Bis(trialkylammoniomethyl)oligodimethylsiloxanes 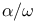 -substituents, interactions of
-substituents, interactions of  -Azido alcohols
-Azido alcohols  -Silylcarbenium ions
-Silylcarbenium ions  -bond energies
-bond energies