|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Patai S. — The Chemistry of Organic Silicon Compounds (part 1-2) |
|
|
 |
| Предметный указатель |
Dimethylsilacyclobutanes 34 971 1053
Dimethylsilanone, photochemical expulsion of 983
Dimethylsilathione, PE spectra of 572
Dimethylsilene gas-phase acidity of 451
Dimethylsilene photochemical expulsion of 983
Dimethylsilene reactivity of 124
Dimethylsilene synthesis of 1053 1057
Dimethylsiloxanes, ring-chain equilibria of 1293
Dimethylsilylene 171 173 174—175 1051
Dimethylsilylene anion 451
Dimmel, D.R. 480 (118) 508
Diorganopolysiloxanes, manufacture of 1290—1291
Diphenhydramine, sila-analogue of 1171
Diphenylcyclosilanes 1208 1209
Diphenylphosphinotrimethylsilane 797
Diphenylsilanediol 1153 1188
Diphosphabuta-1,3-dienes, silyl-substituted 1373
Diphosphasiliranes 262
Diphosphaurea, silyl-substituted 1370
Diphosphines, silyl-substituted 1375 1377 1380
Dipole polarizability, carbon compared with silicon 70 230
Direct process reaction 23—25 1290 1427
Direct process reaction products from 24
Direct pyrolysis-mass spectrometry (DPMS) 1324—1325
Directing effects 893—956
Disilaalkenes see “Disilenes”
Disilabenzenes 155—158
Disilabicyclo[2.2.2]octadiene, disilenes synthesized from 1022—1023
Disilacyclobutadienes 166
Disilacyclobutene, formation during photolysis 974
Disilacyclohexanes 700—702
Disilacyclopropanes formation during photolysis 975 995
Disilacyclopropanes NMR spectra of 536
Disilacyclopropanes photolysis of 980 995
Disilacyclopropanes synthesis of 695
Disilane 78—79
Disilane PE spectra of 587—588 590
Disilanes 249
Disilanes photolysis of 973—975
Disilanes reactions with sulfur 1402
Disilanes Si-Si spin-spin couplings 549
Disilanes substituted, NMR spectra of 544
Disilanes synthesis of 663
Disilanesilylene 136
Disilaoxetanes 695
Disilapyrazine derivatives 703
Disilatetrahedrane 101
Disilatetralin derivatives 702
Disilatetroxacycloalkanes 742
Disilathianes 746—748
Disilene 129 130
Disilene addition of 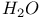 to 134 to 134
Disilene addition of HCl to 134
Disilene hydrogenation reaction of 134
Disilenes 33 34—38 129—136
Disilenes  -bond strength of 131 -bond strength of 131
Disilenes bimolecular reactions of 1037—1044
Disilenes by 4+2 cycloreversion 1022—1024 1029—1031
Disilenes by dimerization of silylenes 1021 1027
Disilenes by irradiation of cyclotetrasilanes 1022
Disilenes by photochemical reactions 1024—1026
Disilenes by photofragmentation of cyclotrisilanes 1022
Disilenes by reduction of 1,2-dihalogenated disilanes 1031
Disilenes by silylsilylene-to-disilene rearrangement 1024—1025 1027—1029
Disilenes by thermal reactions 1026—1031
Disilenes cis-trans isomerization of 979
Disilenes comparison with olefins 35 36—37
Disilenes electrophilic attack on 1041
Disilenes formation during photolysis 977 978 999
Disilenes geometrical isomerization of 1032
Disilenes hydrogen migration in 133 134
Disilenes isolable 1019—1021
Disilenes isomerization to silylenes 1032—1033
Disilenes methyl migration in 136
Disilenes NMR spectra of 532—533
Disilenes nucleophilic addition to 1038
Disilenes nucleophilic cycloaddition to 1038—1039
Disilenes oxidation of 1042
Disilenes pericyclic reactions of 1039—1041
Disilenes photochemical expulsion of 982
Disilenes photochemical isomerization/fragmentation of 1034—1036
Disilenes photochemical reactions of 1043—1044
Disilenes photochemistry of 993
Disilenes pyramidalization at silicon 130
Disilenes radical attack on 1041—1043
Disilenes reactions of 35 134—135
Disilenes reactivity of 1031—1044
Disilenes reductive coupling to 34
Disilenes relation to isomers 131—134
Disilenes silyl migration in 136
Disilenes singlet state of 129—131
Disilenes solution-observable 1021—1024
Disilenes structural parameters for 234 1020—1021
Disilenes substituent transposition in 1033—1034
Disilenes substituted disilenes 135—136
Disilenes synthesis of 34—35 1019—1031 1208
Disilenes thermal isomerization of 1031—1034
Disilenes transient species 1024—1031
Disilenes triplet state of 131
Disilenes [l,5]-sigmatropic shift in 1034
Disiletanes 696
Disiliranes 695
Disilolanes 698
Disilolidines 718
Disiloxane 84 86
Disiloxane PE spectra of 607—609
Disiloxanes, structural parameters of 262 263
Disilthianes 1404
Disilyl-bridged ring systems, photolysis of 981—982
Disilylalkene groups, analytical determination of 417
Disilylamines, structural parameters of 256
Disilylketene, formation during photolysis 992
Disilylsilylene 171
Disilyne bridged form 149
Disilyne linear geometry 148
Disilyne trans-bent structure 149
Disilyne triplet surface 150
Disilynes 147—150 1131
Disodiosilane 80
Disodiosilane square-planar form 212
Dissociative processes 859
Dissociative processes energy barriers to 860—863
Distefano, G. 617 (158) 618 625 191) 626 628 636 638 231 233) 639 640 650 651 652
Ditchfield, R. 63 (31a 31c) 152 214 221
Dithiasilacyclohexane 1406
Dithiatetrasilacyclohexane 1405
Dithiosilane 81—83
Ditsent, Y.Yc. 1335 (180) 1360
Dittmar, G. 265 (287) 300
Divalent silicon 231—233
Divalent state stabilization energy 387—388
Dixon, D.A. 80 83 140 191 (319) 201 216 220 223 224 491 509 822 825 836
Dixon, R.N. 420 (236) 439 604 607 649
Djerassi, C. 455 (54) 459 460 463 481 501 506 507 508 510 1011 1014
Djuric, S. 446 (1c) 504 718 757 1364 1365 1391 1395 1411
Djurovich, P.I. 1230 (107 110) 1239 1461 283) 1476
Dmokhovskaya, Ye.B. 1297 (27) 1357
Do, Y. 1401 1408 1411
Doak, G.O. 1266 (127) 1287
Dobbs, K.D. 12 (75) 51 199 224
Doddrell, D.M. 8 12 551 1309 1358
Dodecamethyl-1,2,9,10-tetrasila[2.2]orthocyclophane 705—706
Dodecamethylcyclohexasilane 1211
Dodecamethylcyclohexasilane radical ions 579—580
Dodecamethylhexasilacyclohexane 977—978
Dodgson, K. 1309 (115) 1359
Dolejsek, Z. 201 (354b) 224
Dolenko, G.N. 875 (101) 891
Doletskaya, T.D. 1297 1299 1300 1357
Dolgaya, M.E. 674 (99) 753 917 918 959
| Dolgy, I.E. 468 (84) 507
Dombrovska, L.E. 1151 1152 1200
Domcke, W. 556 557 558 563 583 585 596 615 644
Domenges, B. 83 84 85 216
Domincone.J.J. 419 (287) 440
Domingas, A.M. 241 (72) 295
Donahue, P.E. 532—534 536 544 549 552 1309 1359
Donaldson, J.D. 130 (209b) 220
Doncaster, A.M. 6 (36a) 50 199 224 372—375 377 380 381 388
Dondini, A. 915 (126) 959
Dondoni, A. 690 (156) 755 764 804
Donnay, G. 7 (45a) 50
Donohue, J. 251 (154) 297 1214 1216 1217 1238
Donovan, P.W. 1420 (45) 1471
Dopamine, silyl derivatives of 1146
Dorfman, E. 402 (72) 435
Dorfmeister, G. 1366 (13 14) 1391
Doris, K.A. 632 633 652
Doron, V. 1257 1267 1287
Dorr, J. 556 557 558 644
Dorrow, A. 399 (51) 435
Dossel, K.F. 259 (222) 299
Dostal, P. 407 (122) 409 436 437
Dotgov, B.N. 420 (321) 440 731 759
Dottore, M.F. 489 (143) 509
Doubek, M. 428 (427) 443
Double-zeta basis sets 63
Doubly bonded silicon compounds see also “Disilenes”; “Silenes”
Doubly bonded silicon compounds structural aspects 234—242
Doubly bonded silicon compounds theoretical aspects 103—145
Doubly charged ions 501—504
Doubly coordinated silicon compounds, PE spectra of 563—568
Doucet, A. 931 (182) 960
Doughcnbough, N.E. 409 (160) 437
Douglas, A.E. 448 (28a) 505
Douglas, J.E. 8 (70) 51
Douglas, W.E. 322 357 367 1443 1444 1474
Doun, S.K. 248 (127) 297
Dousmanis, G. 5 (31) 50
Dousse, G. 572 638 647
Dow Corning Corp. 725 (297 299) 758 1154 1201
Dowd, D.R. 1121 (377) 1141
Dower, D. 198 (337b) 224
Downing, J.W. 102 103 218 1020 1111 1134 1140 1229 1230 1239
Doyle, C D. 1336 (183) 1360
Doyle, D.J. 1051 (168 171) 1086 171) 1137
DPX H6573 1155—1156
Dracke, J.E. 731 (343) 759
Draeger, M. 1214 (38) 1238
Drager, M. 251 (156) 297
Drager, M.A. 1389 (114) 1394
Drago, R.S. 811 (10) 812 835
Drahnak, T.J. 35 (184) 54 114 115 167 173 174 219 221 450 41d) 506 982 991 125) 1003 1004 1024 1046 130) 1075 1082 1100 1134 1136
Drake, J.E. 549 (148) 553 586 602 604 607 639 639 648 750 761 1407 1408 1412
Drake, J.W. 1409 1410 1413
Dramer, K. 34 (181a) 54
Drapailo, A.B. 41 (221) 55 1117 1141
Dreczewski, B. 267 (293 298) 300 301 1219 1238 1405 1412
Dreeskamp, H. 548 (141 154 155) 553 554
Dressier, M. 431 (456) 443
Drewello, T. 197 198 224 456 466 506
Drone, F. 698 (183) 755
Drozdov, V.A. 409 (146—148) 410 174 177—179) 411 181) 412 426 437 438 442
Drozdov, Yu.N. 263 (268) 300
Drugs sila-substitution of 276—279 1159—1183
Drugs silyl derivatives of 1145—1150
Du Mont, W.W. 233 (43) 295 630 652 1367 18 19) 1391 1388 1393 1397 1410 1411 1413
du Pont 681 (135) 754
du Pont de Nemours and Co 1155 (97 98) 1156 104) 1201
Dua, S.S. 884 (134) 886 892 1010 1013
Dual substituent parameter approach 901—902
Dubac, J. 314 (41) 351 353 159) 366 369 750 761 849 34b 35a 36a 36b) 889 940 232) 941 961 1050 157) 1086 157) 1087 157) 1137 1419 1471
Dubchak, I.L. 263 (276 277) 300 1297 1357
Dube, G. 452 (51a—51c) 453 51d) 454 455 471 506
Dubinskaja, E.I. 544 (121) 553
Dubinskaya, E.I. 527 (63) 552
Dubois, I. 168 (270) 173 279) 222 231 295
Duboudin, F. 713 (233) 733 756 759 920 959 1021 1030 1038 1039 1055 1134
Duchesne, J.S. 416 (227) 439
Duckett, J.A. 259 (224) 299
Due Tran Qui 1258 (117) 1287
Dufaut, N. (81) 1201
Duff, B.D.W. 306 307 331 365
Duff, J.M. 328 (83) 367 920 925 942 959 961 980 984 985 986 103 104) 1003
Duffaut, M.I. 1153 (80) 1201
Duffaut, N. 660 (27) 666 671 674 720 745 752 753 757 760 1400 1411
Dukli, I. 727 (320) 758
Dumogues, J. 1400 (21d) 1411
Dumont, W. 942 (241) 961 1407 1412 1508 141) 1510 1511 1524
Duncan, D.P. 696 (173) 755 970 1002 1026 1050 1051 1134 1405 1412 1465 313) 1477
Duncanson, L.A. 95 100 218
Dunitz, J.D. 828 (120) 830 831 837 948 962 1254 1259 1286
Dunlap, N.K. 766 (12) 805
Dunn, G.E. 413 (197) 438 708 756 1010 1013
Dunning, T.H. 62 (28) 214
Dunning, T.H., Jr. 63 (30a) 214
Dunny, S. 1482 (36) 1521
Dunogues, J. 660 (27) 666 671 673 674 678 682 685 720 752 753 754 757 789 907 913 120 121) 915 931 932 935 938 940 941 958 959 960 961
Dupuis, M. 12 (74) 51 106 107 109 111 219
Durand, P. 65 (40) 215
Duranti, F. 715 (247) 757
Durgafyan, S.G. 1298 (35) 1357 1482 1521
Duric, N. 448 (28b) 505
Durig, J. 13 (86) 51
Durig, J.R. 5 14 50 79 86 216 217 246 105) 258 269 319) 296 298 301 353 369
Dusek, R. 424 (381 384) 427 442
Dutheil, J.P. 342 (141) 369 1278 1288
Dutler, R. 90 91 217
Duvall, R.B. 418 (276) 440
Dvoracek, J. 432 (467) 443
Dye, M.J. (152) 1162 1203
Dyke, J.M. 187 188 199 223 556 558 559 563 568 70 73) 570 70) 615 617 645 646 647
Dykema, K.J. 11 (63) 51 138 139 151 180 220 222 260 261 299
Dynamic stereochemistry 305—365
Dynamic stereochemistry hypervalent silicon compounds 1259—1266 (see also “Stereochemistry”)
Dzarnoski, J. 1027 1028 1131 1134
Dzhafarov, A.A. 1482 1483 1521
Dzhaparidze, K.G. 353 (160) 369
Dziedzic, J. 815 (29) 816 835
Dzintara, M. 1461 (275) 1476
d’Angelo, J. 788 (106) 806
E-1,2-Dialkyl-1-trimethylsilylethene 666—667
Eaborn, C. 17 (101) 20 23 26 33 171) 51 52 54 185 223 265 275 377) 300 302 306 307 323 328 330 97 99) 331 111) 365 367 368 413 438 446 3a 5a) 504 505 658 15) 670 76) 677 721 723 744 751 752 753 754 757 760 816 817 820 835 836 840 851 863 865 873 880 116 118 145) 881 882 125—128) 883 130) 884 128 130—136) 885 126 128 137) 886 131 137 138 140—142 144 145 147—149 ) 887 151—153) 888 (153—157) 888 889 890 891 892 894 895 903 50a 50b 75) 904 51 56 77) 905 909 80) 910 85—89 92 99) 911 912 89 92) 913 119) 918 919 956 957 958 959 967 968 995 1001 1004 1010 1013 1062 1070 267) 1077 1081 1086 267) 1124 382) 1138 1139 1141 1267 1274 1279 1287 1288 1342 1360 1395 1396 1400 1410 67) 1410 1411 1413 1420 1442 1443 1444 1445 1459 1466 1471 1474 1477 1480 1481 1485 50a) 1486 1487 1488 1500 1520 1521 1523
Eades, R.A. 201 (351b) 224
Eagle, M.V. 681 (136) 754
Ebata, T. 946 (262) 962
Ebelmen, M. 19 29 52
Ebic, M. 1168 (173) 1203
Ebsworth, E.A.V. 4 (4) 13 85 87—89) 49 51 79 216 242 76) 246 113) 255 166 171 173) 259 263 266—268 275 296 297 298 299 302 340 368 415 416 420 421 341 348) 438 441 518 540 548 551 553 584 586 108) 602 603 604 618 619 620 176) 621 177) 622 629 630 210) 631 216) 632 634 637 639 167) 640 167) 641 642 648 650 651 652 653 677 746 754 760 819 822 831 832 137 138) 834 141) 835 836 837 854 876 890 891 894 895 896 897 956 1244 1260 1285 1417 1434 1441 1470 1473 1474
Ebsworth, E.V.A. 1246 (38) 1253 84) 1255 38 83 84) 1285 1286
Eckberg, R.P. 1346 (220) 1348 1361
Eckert-Maksic, M. 634 (224) 652
Ecknig, W. 419 (297) 440
Ecological impact 1184
Edcr, U. 1156 (100) 1201
Edgcll, R. 556 557 558 583 584 593 595 596 602 615 619 644
Edlund, U. 1008 (4) 1009 1013
Edstrom, E.D. 775 (55) 806
Eduardofit, F. 22 (118) 52 680 754
Edwards, J.D. 1447 (220) 1475
Effenberger, F. 681 685 754 912 916 130) 958 959
Eflendene, B. 674 (103) 754
Efremova, L.A. 412 (184) 414 416 438 439 1493 1495 1522
Egert, E. 257 265 298
Egger, K.W. 6 (34a) 50
Eglen, R.M.M. 1172 (205 206) 1176 1204
Egorochkin, A.N. 430 (445) 443 834 838
Ehler, D.F. 664 (44) 752 1482 1521
Ehlinger, E. 921 (153) 948 949 283) 950 959 962
Ehrcnson, S. 902 (39) 957
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 to
to  -bond strength of
-bond strength of