|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Patai S. Ч The Chemistry of Organic Silicon Compounds (part 1-2) |
|
|
 |
| ѕредметный указатель |
Showatka, K.H. 420 (332) 441
Shrinkage correction 259
Shrinkage correction 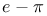 - conjugation see ЂHyperconjugationФ - conjugation see ЂHyperconjugationФ
Shrinkage correction e constants 525 526
Shtifman, L.M. 412 (184) 438
Shubert, D.C. 784 (93 94) 806
Shull, E.R. 414 415 438
ShulyatТeva, T.I. 426 (403) 442
Shumann, C. 548 (141) 553
Shvyrkova, L.A. 406 (119 120) 407 436
Shymanska, M.V. 921 (151) 959
Si-C spin-spin couplings 548
Si-H spin-spin couplings 548 (see also УAlkyl...Ф; УAlkyny...Ф; УAllyl...Ф; УAryl...Ф; УCyclosilanesФ; УDisilanesФ; УOptically active...Ф; УPoly...Ф; УVinylsilanesФ)
Sibermann, J. 334(114) 368
Sibille, S. 682 (140) 754
Sidorkin, V.F. 538 (95) 552
Sidorov, V.D.I. 1493 (83) 1522
Sidorov, V.I. 903 (75) 918 958 959 1515 1524
Siebel, D. 403 404 436
Siebel, G.H.R. 403 404 436
Siegbahn, K. 557 (27 28) 558 28) 615 28) 645
Siegbahn, P.E.M. 205 207 225
Siegel, D.A. 35 (191a) 54 532 552 1019 1020 1033 1133
Sievers, R. 260 (231) 299
Siganama, K. 1234 (122) 1240
Siggia, S. 405 (106) 436
Sigmatropic shifts disilenes 1034
Sigmatropic shifts silenes 1046Ч1049 1061 1066Ч1067 1076Ч1079
Sigwalt, P. 1303 (56 60) 1304 66 68) 1307 79) 1357 1358
Siklosi, M.P. 698 699 705 755
Sila-drugs 276Ч279 1159Ч1183
Silaacetylene 145Ч146
Silaacetylenes see ЂSilynesФ
Silaacyl complexes 1453Ч1458
Silaaldehydes see ЂSilanonesФ
Silaalkenes see ЂSilenesФ
Silaalkynes see ЂSilynesФ
Silaallenes 125 128
Silaallenes dimerization of 1101
Silaallenes formation during photolysis 975
Silaaromatics photochemical generation of 1102
Silaaromatics photochemical reactivity of 1108
Silaaromatics preparation in solution 1106
Silaaromatics reactivity of 1107Ч1108
Silaaromatics synthesis of 1102Ч1106
Silaaromatics thermal generation of 1102Ч1105
Silaaromatics thermal reactivity of 1107Ч1108
Silabenzene 151Ч153
Silabenzene PE spectra of 560Ч563
Silabenzene silylene isomers 152
Silabenzenes 151Ч161
Silabenzenes degree of aromaticity of 152
Silabenzenes disilabenzenes 155Ч158
Silabenzenes hexasilabenzene 158Ч161
Silabenzenes ipso-substituted silabenzenes 155 156
Silabenzenes PE ionization energies of 624Ч627
Silabenzenes reactivity of 1107
Silabenzenes substituted silabenzenes 153Ч155
Silabenzenes synthesis of 1102Ч1106
Silabicyclo[1.1.0]butanes 100Ч101
Silabicyclo[2.2.2]octadienes 1056Ч1057
Silabolin, pharmacological properties of 1147Ч1148
Silabutadienes 125
Silabutadienes electrocyclic interconversion of 1079
Silacyclic compounds, enthalpies of formation of 378
Silacyclobutadiene 165Ч166
Silacyclobutadiene formation during photolysis 998
Silacyclobutane 101
Silacyclobutanes gas-phase reactions of 498
Silacyclobutanes photolysis of 971Ч972
Silacyclobutanes silenes synthesized from 1044 1050 1054Ч1056
Silacyclobutanes synthesis of 695
Silacyclobutenes 128
Silacyclobutenes electrocyclic interconversion of 1079
Silacyclobutenes photolysis of 972
Silacyclobutenes synthesis of 695
Silacyclohexadienes PE spectra of 562
Silacyclohexadienes photolysis of 980
Silacyclohexane derivatives, synthesis of 698
Silacyclopentadienyl anion 162Ч164 203 1010
Silacyclopentenes formation of 969
Silacyclopentenes photochemical reactions of 970Ч971
Silacyclopentenes synthesis of 696
Silacyclopropane 101
Silacyclopropanes 93 245
Silacyclopropanes formation of 974
Silacyclopropanes NMR spectra of 517Ч518 536
Silacyclopropanes photochemical rearrangements of 968Ч970
Silacyclopropanes photolysis of 997
Silacyclopropenes 101 102 126 245
Silacyclopropenes formation during photolysis 974
Silacyclopropenes NMR spectra of 536 537
Silacyclopropenes photochemical reactions of 995
Silacyclopropenium cation 162Ч163
Silacyclpphanes, synthesis of 705Ч706
Silaethene, PE spectrum of 563
Silaethenes see ЂSilenesФ
Silaethylene 106Ч119
Silaethylene  -bond strength in 109Ч112 -bond strength in 109Ч112
Silaethylene addition of 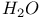 to 117 to 117
Silaethylene addition of HCl to 117 118
Silaethylene addition reactions of 117Ч119
Silaethylene dimerization of 116Ч117
Silaethylene electronic structure of 112Ч113
Silaethylene geometry of 106 108Ч109
Silaethylene higher energy states of 109
Silaethylene hydrogenation of 119
Silaethylene infrared vibrational frequencies of 108Ч109 110
Silaethylene isomerization of 113Ч116
Silaethylene polarity of 112
Silaethylene reactions of 116Ч119
Silaethylene relative stability of 113Ч116
Silaethylene substituted silaethylenes 119Ч123
Silaethylene triplet states of 109
Silaethylenes see ЂSilenesФ
Silaethynes see ЂSilynesФ
Silaimines see ЂSilaniminesФ
Silaindanes, formation of 974
Silaindenes, formation during photolysis 995
Silaisonitriles 150Ч151 1131Ч1133
Silaisonitriles phosphorus analogues of 151
Silaketimine adducts 240
Silaketimines 239Ч240
Silaketones see ЂSilanones
Silalkynes, photochemical reactions of 995
Silamantane, PE spectrum of 616 617
Silamebrobamate derivatives, metabolic fate of 1185
Silameprobamate and derivatives 1159
Silameprobamate and derivatives metabolism of 1185
Silane 78
Silane bond dissociation energies of 388
Silane hydride affinity of 451Ч452
Silane ion 447
Silane PE spectrum of 590
Silane square-planar form 211
Silane tetrahedral form 211
Silanediol 81Ч83
Silanediols, synthesis of 724
Silanephosphimines 41 138Ч139 1116Ч1117
Silanephosphimines inversion at phosphorus atom 139
Silanes ionization energies of 618
Silanes ketones reduced by 776
Silanes nucleophile interactions 876Ч880
Silanes PE spectra of 588Ч593
Silaneselenones 1130Ч1131
Silanethiols 1403Ч1404
Silanethiols acidity of 813
Silanethiols synthesis of 745Ч746
Silanethione 143Ч145
Silanethione  -bond in 143 145 -bond in 143 145
| Silanethione addition of 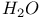 to 145 to 145
Silanethione dimerization of 145
Silanethiones 42 143Ч145 1127Ч1130
Silanetriol 33
Silanimine 137
Silanimines 40Ч41 136Ч138
Silanimines  -bond strength in 137 -bond strength in 137
Silanimines 2+2 cycloaddition to 1114Ч1115
Silanimines 2+3 cycloaddition to 1115
Silanimines ene addition to 1115
Silanimines formation during photolysis 993
Silanimines hydrogenation reaction of 138
Silanimines inversion at nitrogen atom 137
Silanimines isolable silanimines 1109Ч1110
Silanimines nucleophilic addition to 1112Ч1114
Silanimines nucleophilic attack on 1112Ч1115
Silanimines nucleophilic cycloaddition to 1114Ч1115
Silanimines observation in solution 1110Ч1111
Silanimines photochemical generation of 1111Ч1112
Silanimines reactivity of 1112Ч1116
Silanimines structure of 1110
Silanimines synthesis of 1108Ч1112
Silanimines thermal generation of 1112
Silanimines transient species 1111Ч1112
Silanitriles 150Ч151 1131Ч1133
Silanitriles phosphorus analogues of 151
Silanol 79 180
Silanol enthalpy of formation of 381
Silanol square-planar form 211
Silanolate anions, structural parameters of 263 264
Silanols acidity of 812Ч813
Silanols analysis of 409Ч410 419
Silanols basicity of 822
Silanols biological activity of 1152Ч1154
Silanols GC separation of 430
Silanols reactions of 31
Silanols synthesis of 722Ч724
Silanone 140
Silanone addition of 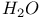 to 142 to 142
Silanone bond strength in 140
Silanone dimerization of 142Ч143
Silanone triplet state of 140
Silanone unimolecular decomposition of 141
Silanones 42 139Ч143
Silanones by cycloreversion 1123Ч1124
Silanones by oxygen atom transfer 1124Ч1126
Silanones by silanolate fragmentation 1124
Silanones formation during photolysis 999
Silanones matrix-isolated silanones 1118Ч1119
Silanones nucleophilic addition to 1126Ч1127
Silanones nucleophilic cycloaddition to 1127
Silanones photochemical generation of 1119Ч1120
Silanones reactions of 141Ч143
Silanones reactivity of 1126Ч1127
Silanones substituted silanones 140Ч141
Silanones synthesis of 1117Ч1126
Silanones thermal generation of 1120Ч1126
Silanones transient species 1119Ч1126
Silanorbornene derivatives NMR spectra of 537 538
Silanorbornene derivatives reactions of 1057 1071
Silaolefins see ЂSilenesФ
Silaoxacycloalkanes 739Ч741
Silaoxaparacyclophanes 742
Silaphosphenes 1383
Silaprocyclidine 1174
Silaprocyclidine metabolic fate of 1188
Silapropadiene, formation during photolysis 974
Silaspiropentanes 102
Silatetrahedrane 101
Silathioketene 129
Silatoluene 151 153
Silatoluene PE ionization energy of 624
Silatoluene PE spectra of 560Ч563
Silatranes 229 279 289Ч290 829
Silatranes biological activity of 1150Ч1152
Silatranes enthalpies of formation of 383
Silatranes NMR spectra of 537Ч538
Silatranes structure of 1244
Silatranes synthesis of 737Ч739
Silatranes toxicity of 1151 1183
Silatranones, NMR spectra of 540
Silazanes NMR spectra of 547
Silazanes synthesis of 709Ч710
Silenate adducts, formation of 1044Ч1045 1083Ч1085
Silene adducts 39 40 238
Silene complexes, transition-metal derivatives 1432Ч1433
Silenes 38Ч40 105Ч129 245
Silenes 1,2-shift of 1072Ч1076
Silenes 2+2 cycloadditions to 1090Ч1093
Silenes 3+2 cycloadditions to 1093Ч1094
Silenes 4+2 cycloadditions to 1090Ч1093
Silenes addition of 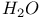 to 123Ч124 to 123Ч124
Silenes addition of HCl to 123Ч124
Silenes addition reactions of 117Ч119
Silenes adducts formed 39 40
Silenes bimolecular reactions of 1083Ч1102
Silenes by 1,2-elimination 1062 1067Ч1070
Silenes by 1,2-shift 1046
Silenes by 2+2 cycloreversion 1050 1063Ч1064
Silenes by 2+3 cycloreversion 1053Ч1056
Silenes by 4+2 cycloreversion 1056Ч1057 1064
Silenes by addition of CO to silylenes 1051
Silenes by dehalogenation 1062
Silenes by dehydrogenation 1062Ч1063
Silenes by electrophilic ring opening 1049Ч1050
Silenes by hydrogen atom abstraction 1050Ч1051
Silenes by pericyclic processes 1061Ч1062
Silenes by photofragmentation of silanes/disilanes 1051
Silenes by pyrolysis 1051Ч1063
Silenes by radical disproportionation/fragmentation 1062
Silenes by rearrangement of carbenes/silylenes 1058Ч1061
Silenes by retro-ene fragmentation 1057Ч1058
Silenes by sigmatropic shifts 1046Ч1049 1061 1066Ч1067
Silenes by silylcarbene-to-silene rearrangement 1060Ч1061 1064Ч1066
Silenes by silylene-to-silene rearrangement 1059Ч1060
Silenes by substituent transposition on alkenes 1070
Silenes by thermal reactions in solution 1063Ч1070
Silenes deprotonation of 1102
Silenes dimerization of 116Ч117 1100Ч1101
Silenes electrocyclic interconversion in 1079
Silenes enereactions of 1079Ч1080
Silenes formation during photolysis 971 973 987 991
Silenes geometrical isomerization of 1070Ч1072
Silenes geometries of 119Ч121
Silenes intramolecular hydrogen atom abstraction reactions of 1080Ч1081
Silenes intramolecular nucleophilic attack in 1081
Silenes isolable 1044Ч1046
Silenes isomerization to silylenes 1072Ч1076
Silenes Lewis basicity towards 39
Silenes metathesis reaction of 143
Silenes NMR spectra of 518 519 520 532Ч533
Silenes nucleophilic addition to 1085Ч1090
Silenes nucleophilic cycloaddition to 1090Ч1094
Silenes nucleophilic ene reactions of 1095
Silenes one-electron reduction of 1036Ч1037
Silenes PE ionization energies of 619Ч620
Silenes pericyclic reactions of 1095Ч1099
Silenes photochemical generation of 1046Ч1051
Silenes photochemical isomerization of 1082Ч1083
Silenes photochemical reactions of 991Ч992
Silenes photochemical synthesis of 971Ч972
Silenes preparation of 38Ч39 1044Ч1045 1046Ч1070
Silenes prochiral silenes 314
Silenes radical attack on 1101
Silenes reactions of 39 40 122Ч124
Silenes reactivity of 1070Ч1102
Silenes rearrangements of 991
Silenes retro-ene reactions of 1079Ч1080
Silenes sigmatropic shifts in 1076Ч1079
Silenes silenate formation from 1083Ч1085
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



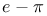 - conjugation
- conjugation  -bond strength in
-bond strength in 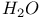 to
to