|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Patai S. Ч The Chemistry of Organic Silicon Compounds (part 1-2) |
|
|
 |
| ѕредметный указатель |
Silenes structural parameters for 236Ч237
Silenes structure of 1045Ч1046
Silenes substituted silenes 119Ч124
Silenes synthesis of 1044Ч1045 1046Ч1070
Silenes thermal isomerization/fragmentation of 1070Ч1081
Silenes thermodynamic stability of 121Ч122
Silenes transient species 1046Ч1070
Silenes unimolecular reactions of 1073
Silenyl anion 203
Siletanes 695
Siletes 695
Silicate anions 282Ч289
Silicenium ions 184Ч193
Silicenium ions alkylsilicenium ions 188 485
Silicenium ions as electrophiles in cyclization 469Ч473
Silicenium ions hydroxy-substituted 188
Silicenium ions phosphino-substituted 187
Silicenium ions rearrangements involving 454Ч465
Silicenium ions silyl-substituted 190
Silicenium ions soivation of 190Ч191
Silicenium ions stabilities of 449
Silicenium ions substituted 187Ч190 448Ч450
Silicenium ions sulfur-substituted 188
Silicide clusters 1423
Silicocations see ЂSilyl cationsФ
Silicocene 162 642
Silicon analytical determination of 394Ч398
Silicon atomic properties of 70 230
Silicon carbide fibers 43 48 1224 1233Ч1234
Silicon clusters 102
Silicon clusters transition-metal derivatives 1423Ч1424
Silicon dihalides, PE spectra of 570Ч571
Silicon dioxide 139
Silicon electronegativity of 895
Silicon halides enthalpies of formation of 389
Silicon halides PE spectra of 602Ч607
Silicon hydride cations, stabilities of 446Ч448
Silicon hydrides as reducing agents 1267 1274Ч1276
Silicon hydrides enthalpies of formation of 389
Silicon hydrides GC retention times for 421 422
Silicon hydrides NMR spectra of 530 531
Silicon isotope used in NMR 521
Silicon manufacture of 19
Silicon monoxide, PE spectrum of 570
Silicon oxide 139
Silicon sources of 18
Silicon tetrahalides, PE spectra of 604Ч607
Silicon uses of element 19
Silicon-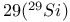 ESR/ENDOR spectroscopy 577 ESR/ENDOR spectroscopy 577
Silicon-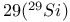 NMR spectroscopy 520Ч550 NMR spectroscopy 520Ч550
Silicon-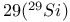 NMR spectroscopy chemical shift ranges listed 523 NMR spectroscopy chemical shift ranges listed 523
Silicon-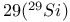 NMR spectroscopy electronegative substituent effect 524 NMR spectroscopy electronegative substituent effect 524
Silicon-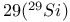 NMR spectroscopy organosilicon polymers 1309 NMR spectroscopy organosilicon polymers 1309
Silicon-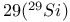 NMR spectroscopy paramagnetic relaxation reagent used 521 NMR spectroscopy paramagnetic relaxation reagent used 521
Silicon-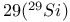 NMR spectroscopy polarization transfer used 521 522 NMR spectroscopy polarization transfer used 521 522
Silicon-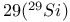 NMR spectroscopy polysilane polymers 1232Ч1233 NMR spectroscopy polysilane polymers 1232Ч1233
Silicon-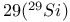 NMR spectroscopy reference compounds used 522Ч523 NMR spectroscopy reference compounds used 522Ч523
Silicon-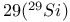 NMR spectroscopy solid-state NMR studies 544 NMR spectroscopy solid-state NMR studies 544
Silicon-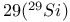 NMR spectroscopy spin-lattice relaxation 521 549Ч550 NMR spectroscopy spin-lattice relaxation 521 549Ч550
Silicon-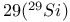 NMR spectroscopy spin-spin couplings 547Ч549 NMR spectroscopy spin-spin couplings 547Ч549
Silicon-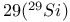 NMR spectroscopy transition-metal silyl complexes 1439 1440Ч1441 NMR spectroscopy transition-metal silyl complexes 1439 1440Ч1441
Silicon-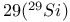 NMR spectroscopy УsaggingФ pattern of chemical shifts 524 NMR spectroscopy УsaggingФ pattern of chemical shifts 524
Silicon-bridged ring systems, photolysis of 981Ч984
Silicon-silicon bonds 249Ч254
Silicon-silicon bonds angles of 254
Silicon-silicon bonds dissociation energies of 385
Silicon-silicon bonds interactions, PE spectra affected by 587Ч593
Silicon-silicon bonds lengths of 249 251 1020
Silicon-silicon double bonds 234Ч236
Silicon-silicon double bonds length of 129Ч130
Silicon-X bonds bond dissociation energies of 77
Silicon-X bonds lengths of 71 74 1245
Silicon-X bonds strengths of 74Ч78
Silicon-X double bonds 103
Silicon-X multiple bonds in neutral compounds 481Ч483
Silicon-X multiple bonds ions containing 475Ч481
Siliconate anions, pentacoordinated form of 1265
Silicone oils, NMR spectra of 1318
Silicone polymers 31
Silicone polymers analysis of 410 413 415 419
Silicone polymers formation of 31 1013
Silicone polymers NMR spectra of 545Ч547 1318
Silicone polymers toxicological behavior of 1184
Silicones see also ЂOrganofunctional siloxanesФ
Siliconic acids 33
Siliconium ions, pentacoordinated 191
Silidyne groups, analysis of 406Ч407
Silines 698
Siliranes 694
Silirenes 694
Silkina, N.N. 483 (123) 508
Silolanes 697
Silole derivatives 696 697
Silolenes 696
Silolidines 718
Siloxane copolymers air-liquid interface behavior of 1354Ч1355
Siloxane copolymers composition of 1313Ч1314
Siloxane copolymers degradation of 1333Ч1335
Siloxane copolymers direct (thermodynamic) analysis of 1316Ч1319
Siloxane copolymers kinetic-chain analysis for 1315
Siloxane copolymers macrostructure of 1311Ч1314
Siloxane copolymers microstructure of 1314Ч1319
Siloxane copolymers number average sequence length of 1315Ч1316
Siloxane groupings, analysis of 415
Siloxane polymers degradation of 1319Ч1341
Siloxane polymers molecular weights of 1301
Siloxane polymers NMR spectra of 545Ч547 1318
Siloxane polymers redistribution reactions in 1301Ч1302
Siloxane polymers surface activity of 1351Ч1354 1355Ч1356
Siloxane polymers toxicological behavior of 1184
Siloxanes analysis of 394 395 398 406 408 409 418Ч419
Siloxanes basicity of 822
Siloxanes biological activity of 1154Ч1155
Siloxanes enthalpies of formation of 381Ч382
Siloxanes GC separation of 428Ч429
Siloxanes IR analysis of 418Ч419
Siloxanes NMR spectra of 534Ч536
Siloxanes ring-chain equilibria of 1292Ч1294
Siloxanes synthesis of 724Ч725 (see also УCyclosiloxanesФ; УOrganofunctional siloxanesФ; УPolysiloxanesФ)
Siloxetenes, formation during photolysis 990
Siloxiranes, formation during photolysis 977
Siloxthianes 1402
Siloxy transfer 460
Siloxycarbenes, formation during photolysis 985
Silthianes cage silthianes 1401
Silthianes cyclic silthianes 1401Ч1403
Silthianes linear silthianes 1400Ч1401
Silver, J.M. 130 (209b) 220
Silver, S.M. 945 (267) 962
Silverman, G. 188 (316) 223 449 506
Silverman, M.A. 309 351 366
Silyamines, acidity of 814
Silyisilylenium ion 190
Silyl acetate 831
Silyl acetate derivatives 458
Silyl anion reagents 1420 1424
Silyl anions 201Ч204
Silyl anions barrier to inversion of 1008
Silyl anions configurational stability of 1008Ч1009
Silyl anions lone-pair conjugation in 1009Ч1010
Silyl anions NMR spectra of 514
Silyl anions nomenclature of 1007
Silyl anions PE spectrum of 1008
Silyl anions preparation of 450 1007Ч1008
Silyl anions pyramidal inversion of 1008
Silyl anions reaction with imines 951
Silyl anions reactivity of 495
Silyl anions structure of 1008
Silyl anions substituted 201Ч204
Silyl arenes, GC separation of 430
| Silyl arsines 1387Ч1389
Silyl bismuthines 1390
Silyl cations nomenclature of 1010
Silyl cations preparation of 1010Ч1011
Silyl cations structure of 1011Ч1013 (see also УSilylenium ionsФ)
Silyl chloride 180
Silyl chloride hydride attack on 208
Silyl enol ethers 732Ч737 1452 1467 1470
Silyl ester migration 469
Silyl esters as reagents 801Ч802
Silyl esters hydrolysis of 887Ч888
Silyl ethers basicity of 826
Silyl ethers GC separation of 429
Silyl ethers PE ionization energies of 636Ч639
Silyl fluoride 180
Silyl fluoride hydride attack on 208
Silyl Grignard reagents 330 1443
Silyl groups, electronic effects of 895Ч899
Silyl halides, PE ionization energies of 639Ч640
Silyl isocyanate 832 833
Silyl nitronates 830Ч831
Silyl peroxides, as reagents 802Ч803
Silyl radicals 198Ч200
Silyl radicals abstraction of hydrogen by 200
Silyl radicals PE spectra of 571
Silyl stibines 1389Ч1390
Silyl sulfides GC separation of 429
Silyl sulfides PE ionization energies of 636 638
Silyl thioacetate 832
Silyl thioethers, GC separation of 429
Silyl trioxides, as reagents 802
Silyl-nitrogen-X fragments, structural parameters of 258
Silyl-oxygen double bond, strength of 140
Silyl-oxygen-X fragments, structural parameters of 263
Silyl-substituted pentacoordinated silicon anions 206
Silyl-substituted phenyl cation 197
Silylacetylene 126 128 203
Silylacetylene radical cation, hyperconjugation in 593Ч596
Silylacetylenes see ЂSilynesФ
Silylamine 79 180
Silylamines basicity of 817 819Ч822 825
Silylamines bond angles of 13
Silylamines enthalpies of formation of 382Ч383
Silylamines IR analysis of 421
Silylamines PE ionization energies of 628Ч631
Silylamines proton affinities of 820Ч822
Silylamines structural parameters of 254Ч256
Silylamines synthesis of 1515Ч1516
Silylated penta-3,4-diene-l-ynes 680
Silylation methods nitrogen-containing compounds 715
Silylation methods oxygen-containing compounds 742Ч744
Silylbarbarlane, photolysis of 981
Silylcarbenes formation during photolysis 989 990
Silylcarbenes siienes synthesized from 1060Ч1061
Silylcarbonylation 1518Ч1520
Silyldiazoalkanes, photolysis of 989Ч990
Silyldisilene 89 136 172
Silylene 167Ч169
Silylene addition to ethylene 182
Silylene complexes 42 180 183
Silylene complexes indirect evidence for 1427Ч1429
Silylene complexes synthesis attempted for 1429Ч1432
Silylene dissociation of 182
Silylene fluorosilane 180
Silylene heat of formation of 168Ч169
Silylene hydrogen 176Ч179
Silylene hydrogen abstraction by triplet silylene 181
Silylene hydrogen chloride 180
Silylene hydrogen fluoride 180
Silylene hydrogen sulfide 180
Silylene insertion into ammonia 180
Silylene ion 448
Silylene methane 178
Silylene phosphine 180
Silylene silanc 178
Silylene singlet-triplet energy difference 167Ч168
Silylene water 180
Silylenes 35 167Ч184
Silylenes cyclic 185
Silylenes dimerization of 175Ч176 977 1021 1027
Silylenes enthalpies of formation of 387
Silylenes expulsion from cyclic di-/tri-silenes 979
Silylenes formation during photolysis 978 998
Silylenes insertion reactions of 176Ч183
Silylenes ionic species 483
Silylenes multisilylenes 185
Silylenes noncyclic 185
Silylenes PE spectra of 563Ч568
Silylenes reactions of 175Ч183
Silylenes rearrangement to disilenes 1024Ч1025 1027Ч1029
Silylenes silenes synthesized from 1059Ч1060
Silylenes singlet-triplet energy differences 167Ч173
Silylenes stereochemistry of expulsion process 979Ч980
Silylenes substituent effects 169Ч170
Silylenes substituted silylenes 169Ч173
Silylenes synthesis of 976
Silylenes thermochemistry of 377
Silylenes UV-visible spectra of 173Ч175 (see also УTransition-metal silylene compoundsФ)
Silylenium cation 186
Silylenium cation heat of formation of 186Ч187
Silylenium ions 184Ч193
Silylenium ions heat of formation of 191
Silylenium ions preparation of 1010Ч1011
Silylenium ions structure of 1011Ч1013
Silylethylenes see ЂSilenesФ
Silylethynes see ЂSilynesФ
Silylidene 146Ч147
Silylisonitrile 80
Silyllithium derivatives 80 269 270 636 787Ч789 1425
Silylmercury derivatives 269 270Ч272 330 540 636 641 1440
Silylmethanol 80
Silylmethyl cation 194
Silylmethyl ketone 196
Silylmethylene 113
Silylnitrile 80
Silylolefins see ЂSilenesФ
Silyloxazoles 690
Silyloxysilaethylene 119Ч123
Silyloxysilene 86
Silylphosphine 79 180
Silylphosphines acidity of 814
Silylphosphines reactions of 1365Ч1369
Silylphosphines structural parameters of 260
Silylphosphines synthesis of 1364
Silylphosphinometal derivatives 1384Ч1387
Silylpolyphosphines 1375Ч1376
Silylpolysulfides 1404
Silylpyridazines 693
Silylpyridines 692Ч693
Silylquinolizines 693Ч694
Silylsiiaethylene 119Ч123
Silylsilanone 140Ч141
Silylsilylene 131Ч133 172 178
Silynes 145Ч147 245
Silynes NMR spectra of 518
Silynes PE ionization energies of 620Ч622
Silynes synthesis of 677
Simchen, G. 682 693 734 754 759 800(152) 807
Simha, R.f. 1315 (141) 1359
Simhon, E.D. 1401 1408 1411
Simon, A. 48 (281) 56 246 249 296
Simon, G.L. 273 (346) 302
Simon, I. 419 (312) 440
Simonis, A.M. 1160 (143) 1202
Simons, J.H. 432 (411) 444
Simpson, C.C. 421 422 441
Simpson, J. 273 (344 345 349) 302 1418 1420 48 49) 1423 1434 134 135) 1435 1437 135) 1470 1471 1473
Simpson, K.A. 272 290 291 301 302 327 367 1437 1473
Simpson, P. 330 (97 99) 367
Simpson, R.N.F. 275 (369) 302 1434 1473
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



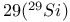 ESR/ENDOR spectroscopy
ESR/ENDOR spectroscopy