|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
 -Ribofuranosyluracil see УUridineФ -Ribofuranosyluracil see УUridineФ
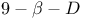 -Ribofuranosyladenine see УAdenosineФ -Ribofuranosyladenine see УAdenosineФ
 mechanism 378 mechanism 378
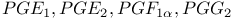 and and 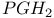 see УIcosanoidsФ see УIcosanoidsФ
 33 (see also УAcidityФ) 33 (see also УAcidityФ)
 mechanism 160Ч161 180 339Ч341 356 mechanism 160Ч161 180 339Ч341 356
 mechanism 163Ч165 181 330Ч336 356 mechanism 163Ч165 181 330Ч336 356
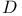 -Glucopyranose 1037Ч1038 1040 -Glucopyranose 1037Ч1038 1040
$\alpha in hydration of alkynes 379Ч381 385
$\alpha in racemization of (R)-sec-butyl phenyl ketone 769
$\alpha of purines and pyrimidines 1156Ч1158 1167 1186
 and and  -Ionone 1107 -Ionone 1107
 -Halo aldehydes, preparation of 757 -Halo aldehydes, preparation of 757
 -Halo carboxylic acids nucleophilic substitution in 816 -Halo carboxylic acids nucleophilic substitution in 816
 -Halo carboxylic acids preparation of 815Ч816 823 -Halo carboxylic acids preparation of 815Ч816 823
 -Halo carboxylic acids reaction with ammonia 816 928 -Halo carboxylic acids reaction with ammonia 816 928
 -Halo ketones, preparation of 757 782 -Halo ketones, preparation of 757 782
 -Helix 1143Ч1145 -Helix 1143Ч1145
 -Keratin 1144 -Keratin 1144
 -Ketoglutaric acid 1123Ч1125 -Ketoglutaric acid 1123Ч1125
 -Olefins see УLinear -Olefins see УLinear  -olefinsФ -olefinsФ
 -Phellandrene 1086 -Phellandrene 1086
 -Pinene 187 1090 -Pinene 187 1090
 -Pinene hydroboration-oxidation of 252 -Pinene hydroboration-oxidation of 252
 -Pinene hydrogenation of 235 -Pinene hydrogenation of 235
 -Santonin 1104 -Santonin 1104
 -Selinene 1085 1086 -Selinene 1085 1086
 -Terpineol 1089 -Terpineol 1089
 Unsaturated aldehydes and ketones conjugate addition to Unsaturated aldehydes and ketones conjugate addition to
 -Alanine 1110 -Alanine 1110
 -Carotene 729 1086 1101 -Carotene 729 1086 1101
 -Keto acids, decarboxylation 818Ч819 824 893 895Ч896 905 -Keto acids, decarboxylation 818Ч819 824 893 895Ч896 905
 -Keto esters acidity of 886 -Keto esters acidity of 886
 -Keto esters alkylation of 894Ч896 907 -Keto esters alkylation of 894Ч896 907
 -Keto esters Michael addition of 901Ч902 -Keto esters Michael addition of 901Ч902
 -Keto esters nomenclature of 887 -Keto esters nomenclature of 887
 -Keto esters preparation of by acylation of ketones 892Ч893 906 -Keto esters preparation of by acylation of ketones 892Ч893 906
 -Keto esters preparation of by Claisen condensation 887Ч890 906 -Keto esters preparation of by Claisen condensation 887Ч890 906
 -Keto esters preparation of by Dieckmann reaction 890Ч891 906 -Keto esters preparation of by Dieckmann reaction 890Ч891 906
 -Keto esters preparation of by mixed Claisen condensation 891Ч892 906 -Keto esters preparation of by mixed Claisen condensation 891Ч892 906
 -Pinene 1090 -Pinene 1090
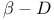 -Glucopyranose 1037Ч1038 1040 1062 -Glucopyranose 1037Ч1038 1040 1062
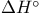 and bond dissociation energy 174 and bond dissociation energy 174
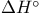 and heats of reaction 84 and heats of reaction 84
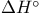 relation to free energy 122Ч123 relation to free energy 122Ч123
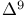 -Tetrahydrocannabinol 1001 1074 -Tetrahydrocannabinol 1001 1074
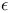 -Caprolactam 861 -Caprolactam 861
 -Aminobutyric acid 1110 -Aminobutyric acid 1110
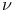 (symbol for frequency) 520 (symbol for frequency) 520
(Benzene)tricarbonylchromium 609
(E)- and (Z)-Cyclodecene 213
(E)-1,2-Dichloroethene, plane of symmetry in 286
(R)-and (S)-2-Bromooctane, stereochemistry of hydrolysis of 331Ч333 343Ч344
(S)-Malic acid 299Ч300
(S)-Malic acid as resolving agent 311Ч312
(Trifluoromethyl)benzene, nitration of 488Ч489 492Ч494
1, 3-Cyclooctadiene, UV-VIS spectrum 565
1,1-Dimethylethyl group 74
1,2-Benzenedicarboxylic acid 792
1,2-Dibromocyclopropane, stereoisomers of 304
1,2-Dibromoethane 257
1,2-Dimethoxyethane 666
1,2-Epoxycyclohexane hydrolysis of 683 684
1,2-Epoxycyclohexane preparation of 677
1,2-Epoxycyclohexane reactions of with hydrogen bromide 683
1,2-Epoxycyclohexane with sodium azide 931
1,2-Epoxypropane chirality center in 285 297
1,2-Epoxypropane preparation of 678
1,2-Epoxypropane reaction with phenylmagnesium bromide 681
1,2-Ethanediol see УEthylene glycolФ
1,3- and 1,4-Pentadiene, relative stabilities 399Ч400
1,3-Butadiene  -molecular orbitals 413-414 -molecular orbitals 413-414
1,3-Butadiene addition of halogens to 407 418
1,3-Butadiene addition of hydrogen halides to 405Ч407 417Ч418
1,3-Butadiene conformations 401Ч402
1,3-Butadiene Dieis Ч Alder reactions of 409Ч414
1,3-Butadiene electrostatic potential map 390
1,3-Butadiene industrial preparation of 404
1,3-Butadiene polymers of 408Ч409
1,3-Butadiene structure and bonding 400Ч402
1,3-Diaxial repulsion 121
1,3-Dihydroxyacetone 1064
1,3-Dihydroxyacetone phosphate 1058
1,3-Diketones acidity of 764Ч765
1,3-Diketones alkylation of 779 781 784
1,3-Diketones enolization of 761Ч762
1,3-Diketones preparation of 892
1,3-Propadiene see УAlleneТТ
1,4-Benzenedicarboxylic acid 806
1,4-Benzenedicarboxylic acid, condensation polymers of 868
1-Aminocyclopropanecarboxylic acid in ethylene biosynthesis 189 1110
1-Bromobutane 152 242
1-Bromobutane alkylation of acetoacetate 895
1-Bromobutane alkylation of acetylene 370Ч371
1-Bromobutane alkylation of o-nitrophenol 1017
1-Bromobutane nucleophilic substitution in 347
1-Butanol acid-catalyzed ether formation from 635 671
1-Butanol conversion to 1-bromobutane 152
1-Butanol dehydration 211
1-Butanol Fischer esterification of 847
1-Butene 188 192
1-Butene addition of hydrogen bromide to 237 242
1-Butene addition of sulfuric acid to 272
1-Butene boiling point 708
1-Butene dipole moment of 196
1-Butene electrostatic potential map 707
1-Butene heat of combustion 197
1-Butene heat of hydrogenation 231Ч233
1-Butyne 364 371
1-Chloro-2, 4-dinitrobenzene, nucleophilic substitution in 976
1-Chloro-2-methylpropane 177 (see also УIsobutyl chlorideФ)
1-Chlorobutane 175Ч176
1-Chloropentane, 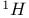 and and 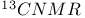 548 550 548 550
1-Decanol 250Ч251 710
1-Decene hydroboration-oxidation of 250Ч251 626
1-Decene hydroxylation of 635
1-Dodecene, epoxidation of 261
1-Fluoro-2,4-dinitrobenzene 976 1131Ч1132
1-Heptanol oxidation of 642
1-Heptanol reaction with hydrogen bromide 152
1-Hexene addition of bromine 273
1-Hexene cis-3-Hexene, reaction of, with hydrogen bromide 236
1-Hexene heat of hydrogenation 233
1-Hexene infrared spectrum 561Ч562
1-Hexynylmagnesium bromide 598
1-Methylcyclopentene addition of hydrogen chloride 237
1-Methylcyclopentene hydroboration-oxidation 252Ч254
1-Methylethyl group 74 (see also УIsopropyl groupФ)
1-Methylpropyl group 74 (see also Уsec-Butyl groupФ)
1-Naphthol, azo coupling of 950
1-Pentanol esterification 656
1-Pentanol reaction with thionyl chloride 180
1-Phenylethylamine, resolution 311Ч312
18-Electron rule 608
2,2,2-Trifluoroethanol acidity of 40Ч41
2,2,4-Trimethylpentane 266
2,2,4-Trimethylpentane, chlorination of 186
2,2-Dimethylpropane 82
2,2-Dimethylpropane chemical shifts (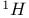 and and 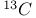 ) 549 ) 549
2,2-Dimethylpropyl group 75
2,3-Butanediol, stereoisomers 303Ч304
2,3-Dihydroxybutanoic acid, stereoisomers of 300Ч302
2,3-Dimethyl-2-butene 207 208Ч209
2,3-Dimethyl-2-butene heat of hydrogenation 233
2,3-Dimethyl-l-butene 207 208Ч209
2,3-Pentadiene, enantiomers 403
2,4,5-T see У2 acidФ
2,4,5-Trichlorophenol 1009Ч1010
2,4,5-Trichlorophenoxyacetic acid 1009Ч1010
2,4-Dinitrophe nylhydr azine 727
| 2,4-Pentanedione  of 36 764Ч765 of 36 764Ч765
2,4-Pentanedione  -alkylation of 781 -alkylation of 781
2,4-Pentanedione enol content of 761Ч762
2-Bromo-2-methylbutane elimination reactions 212 218
2-Bromo-2-methylbutane substitution versus elimination in 349Ч350
2-Bromo-3-methylbutane, rearrangement in hydrolysis of 344Ч345
2-Bromobutane 146 237
2-Bromobutane alkylation of diethyl malonate 898Ч899
2-Bromobutane preparation of 152 354
2-Butanol see also Уsec-Butyl alcoholФ
2-Butanol chirality center in 284 290
2-Butanol enantiomers 289Ч291
2-Butanol reaction with hydrogen bromide 153 354
2-Butanone enolization of 761
2-Butanone heat of combustion 708
2-Butanone proton magnetic resonance spectrum 739
2-Butyne 364 371
2-Chloro-1,3,5-trinitrobenzene 976
2-Chloro-2-methylpropane 177 (see also Уtert-Butyl chlorideФ)
2-Chlorobutane 175Ч176
2-Deoxy-D-ribose 1042 1064 1159Ч1160 1187
2-Heptanone 388 895
2-Methyl-2-butanol dehydration of 204
2-Methyl-2-butanol preparation of 247
2-Methyl-2-butene acid catalyzed hydration 247 626
2-Methyl-2-butene hydroboration-oxidation 251
2-Methyl-2-butene hydrogenation of 231
2-Methyl-2-butene preparation of 204 212 218
2-Methyl-2-butene reaction of with hydrogen bromide 245
2-Methyl-2-butene reaction of with hydrogen chloride 237Ч238
2-Methyl-2-propanol 152 (see also Уtert-Butyl alcoholФ)
2-Methyl-2-propanol acid-catalyzed dehydration 203
2-Methylbutane 82 (see also УIsopentaneФ)
2-Methylcyclohexanol, dehydration of 204
2-Methylpentane 72
2-Methylpentane  of 36 of 36
2-Methylpentane 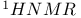 739 739
2-Methylpentane bromation of 177
2-Methylpentane reaction with tert-butylamine 744
2-Methylpropanal acidity of 764
2-Methylpropane 73 (see also УIsobutaneФ)
2-Methylpropane acidity of 593
2-Methylpropane bond dissociation energies in 170Ч171 439
2-Methylpropane chemical shifts carbon 549
2-Methylpropane chemical shifts proton 527 549
2-Methylpropane chlorination 177
2-Methylpropene see also УIsobuteneФ; УIsobutyleneФ
2-Methylpropene addition of hydrogen bromide to 237
2-Methylpropene addition of methanol to 672
2-Methylpropene bromohydrin formation 259
2-Methylpropene dimerization 266
2-Methylpropene dipole moment 196
2-Methylpropene heat of combustion 197
2-Methylpropene hydration mechanism 248
2-Methylpropene preparation of 203
2-Methylpropyl group 74 (see also УIsobutyl groupФ)
2-Naphthol, nitrosation of 1003
2-Octanol 355 596
2-Phenyl-2-butanol 600 640
2-Phenylethanol 639
2-Phenylethanol 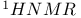 spectrum 653 spectrum 653
2-Phenylpropene hydroxylation of 654
2-Propanol 145 (see also УIsopropyl alcoholФ)
3,3-Dimethyl-1-butene 209
3,3-Dimethyl-2-butanol dehydration and rearrangement of 208Ч210
3-Aminopropanoic acid see У -AlanineФ -AlanineФ
3-Heptanone 740
3-Methyl-2-butanol preparation of 251
3-Methyl-2-butanol reaction with hydrogen chloride 355
3-Methyl-2-butenyl pyrophosphate see УDimethylallyl pyrophosphateФ; УIsopentenyl pyrophosphateФ
3-Methylpentane 73
3-Pentanol, dehydration 206
3-Pentanone cyanohydrin 743
3-Pentanone mass spectrum 741
4-Aminobutanoic acid see ЂУ -Aminobutyric acidФ -Aminobutyric acidФ
4-tert-Butylcyclohexyl bromide, rate of elimination of cis and trans isomers 216Ч217
6-Mercaptopurine 1158
Abscicic acid 1086
Absolute configuration 289Ч293 316
Absorption of electromagnetic radiation 521
Absorption of electromagnetic radiation in infrared spectroscopy 559
Absorption of electromagnetic radiation in nuclear magnetic resonance spectroscopy 522Ч524
Absorption of electromagnetic radiation in ultraviolet-visible spectroscopy 565Ч566
Absorptivity see УMolar absorptivityФ
Acetaldehyde 704
Acetaldehyde bond angles 706
Acetaldehyde enolization of 760
Acetaldehyde formation of in biological oxidation of ethanol 645Ч647
Acetaldehyde in Strecker synthesis of D,L-alanine 1121
Acetaldehyde preparation of by hydration of acetylene 381
Acetaldehyde preparation of from ethylene 269 644
Acetaldehyde reactions of aldol addition 769
Acetaldehyde reactions of hydration 714
Acetaldehyde reactions of with hexylmagnesium bromide 596
Acetaldol 769
Acetals 720Ч724 744
Acetals as protecting group 723Ч724
Acetals glycosides as 1044
Acetals hydrolysis of 722Ч723 724
Acetals preparation of 720Ч723 744
Acetamide 833
Acetanilide 933
Acetanilide preparation and nitration of 941Ч942
Acetanilide reduction of 933
Acetanilide resonance in 940
Acetic acid 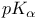 of 36 43 of 36 43
Acetic acid acidity of 43 795Ч797 801 803
Acetic acid conversion to mevalonic acid 1087 1091
Acetic acid electrostatic potential maps acetate ion 796 797
Acetic acid electrostatic potential maps acid 794 797
Acetic acid esterification of 638 656
Acetic acid industrial preparation and use of 806 841
Acetic acid natural occurrence of 4 791 806
Acetic acid natural products derived from 1069Ч1108
Acetic anhydride 831 833
Acetic anhydride in Friedel Ч Crafts acylation 486 502 504 510 842
Acetic anhydride preparation of 841
Acetic anhydride reactions of with  -D-glucopyranose 1058 -D-glucopyranose 1058
Acetic anhydride reactions of with alcohols 656 843 847
Acetic anhydride reactions of with arylamines 843 940
Acetic anhydride reactions of with glycine 1123
Acetic anhydride reactions of with phenols 1004Ч1006 1017
Acetic anhydride reactions of with salicylic acid 1006
Acetic anhydride reactions of with sucrose 1064
Acetic anhydride UV absorption 873
Acetoacetic ester synthesis 894Ч896 907
Acetoacetyl acyl carrier protein 1076
Acetoacetyl coenzyme A 1076 1091
Acetone as solvent 329
Acetone bond angles 706
Acetone enolization of 758 760
Acetone enolization of electrostatic potential map 755
Acetone reactions of aldol condensation 773
Acetone reactions of bromination 758Ч759
Acetone reactions of cyanohydrin formation 719
Acetone reactions of hydration 714
Acetone reactions of reductive amination of 957
Acetone reactions of Wittig reaction 744
Acetophenone 432 486 706
Acetophenone  of 37 of 37
Acetophenone acidity of 764
Acetophenone acylation of enolate 892
Acetophenone phenylhydrazone 727
Acetophenone reactions of aldol condensation 775
Acetophenone reactions of bromination 504
Acetophenone reactions of chlorination 505
Acetophenone reactions of nitration 505
Acetophenone reactions of with butyllithium 627
Acetophenone reactions of with ethylmagnesium bromide 600
Acetyl chloride 831 833
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 -Ribofuranosyluracil
-Ribofuranosyluracil  -Ribofuranosyladenine
-Ribofuranosyladenine  mechanism
mechanism 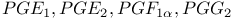 and
and 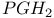
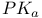
 mechanism
mechanism  mechanism
mechanism 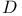 -Glucopyranose
-Glucopyranose  and
and  -Ionone
-Ionone 
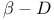 -Glucopyranose
-Glucopyranose 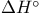 and bond dissociation energy
and bond dissociation energy 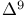 -Tetrahydrocannabinol
-Tetrahydrocannabinol 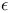 -Caprolactam
-Caprolactam  -Aminobutyric acid
-Aminobutyric acid 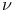 (symbol for frequency)
(symbol for frequency)  -molecular orbitals
-molecular orbitals 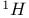 and
and 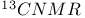
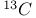 )
)  of
of 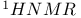
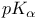 of
of