|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
Chromatography 572Ч573 1130
Chromic acid oxidation of alcohols 641Ч643 657 710 807
Chromic acid oxidation of alkylbenzenes 441Ч442 466 807
Chromic acid oxidation of phenols 1012
Chromophore 567
Chrysanthemic acid 80 1105
Chymotrypsin 1130
Cicutoxin 364
Cimetidine 461
Cinnamaldehyde 193
CIP see УCahn Ч Ingold Ч PrelogФ
Cis and trans descriptors of stereochemistry 124 135Ч136 192Ч193 220
cis and trans-2-Butene 192Ч193
cis and trans-2-Butene, dipole moments of 196
cis and trans-2-Butene, heats of combustion 197
cis and trans-2-Butene, heats of hydrogenation 231Ч233
cis- and trans-1,4-Dimethylcyclohexane 125Ч127
cis- and trans-Decalin 131
cis- and trans-l,2-Dimethylcyclohexane 126 127
cis- and trans-l,2-Dimethylcyclopropane 124Ч125
cis- and trans-l,3-Dimethylcyclohexane 126 128 135Ч136
cis-9-Tricosene 388
Citral 709 1086
Citric acid 324 828
Citronellal 1092
Citronellol 625
Claisen condensation 887Ч890 906
Claisen condensation intramolecular see УDieckmann reactionФ
Claisen condensation mixed 891Ч892 906
Claisen rearrangement 1011Ч1012 1018
Claisen Ч Schmidt condensation 775 783
Claisen, Ludwig 887
Clathrate 66
Clemmensen reduction 486Ч488 505 713
Cocaine 924
Codon 1175Ч1176 1178 1188
Coenzymes 1147 1150
Coenzymes acetyl coenzyme A 1070Ч1071 1091
Coenzymes coenzyme 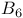 728 728
Coenzymes coenzyme 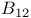 611 611
Coenzymes coenzyme Q see УUbiquinoneФ
Coenzymes heme 1147Ч1148
Coenzymes NAD, NAD +, NADH, NADPH see УNicotinamide adenine dinucleotideФ
Cofactors see УCoenzymesФ
Coke 363
Collins, Francis S. 1182
Columbus, Christopher 408
Combinatorial synthesis 1142
Combustion of alkanes 83Ч87 98
Common names see УNomenclatureФ
Concerted reaction 153
Concerted reaction and orbital symmetry 414Ч415
Concerted reaction bimolecular elimination 214Ч217 221Ч223
Concerted reaction bimolecular nucleophilic substitution 163Ч165 330Ч336 356
Concerted reaction Dieis Ч Alder reaction 409
Condensation polymers 868Ч869
Condensation reaction 635
Condensation reaction aldol 769Ч775 783
Condensation reaction Claisen 887Ч890 906
Condensation reaction Claisen Ч Schmidt 775 783
Condensation reaction ether formation 635 637Ч638 656 671Ч672 693
Condensation reaction Fischer esterification 638Ч639 640 656 810Ч813 823 847
Condensed structural formulas 21 68
Configuration absolute and relative 289Ч290 316
Configuration and Fischer projections 293-295 316
Configuration cis and trans 124
Configuration D-L 1027Ч1032 1061
Configuration erythro and threo 301Ч302
Configuration notational systems  and and  1034 1160 1034 1160
Configuration of aldoses 1031
Configuration of alkenes cis and trans 192Ч193 201 220
Configuration of alkenes E and Z 193Ч195 201 220
Configuration of disubstituted cycloalkanes, cis and trans 124Ч129 135Ч136
Configuration R-S 290Ч293 316
Conformation and nuclear magnetic resonance spectroscopy 545
Conformation chiral 305
Conformation eclipsed 105 106 133
Conformation of 1,3-butadiene 401Ч402 417Ч418
Conformation of heterocyclic compounds 131Ч132 667
Conformation of hydrogen peroxide 104
Conformation pyranose forms of carbohydrates 1038Ч1039
Conformation staggered 105Ч107 133
Conformation(s) 104
Conformational analysis see УConformationФ
Conformations of alkanes butane 109Ч112 133
Conformations of alkanes ethane 105Ч109 133
Conformations of alkanes higher alkanes 110Ч112 134
Conformations of cycloalkanes 114Ч129 134Ч136
Conformations of cycloalkanes cyclobutane 114 134
Conformations of cycloalkanes cyclohexane and derivatives 116Ч124 125Ч129 134Ч136 305 545
Conformations of cycloalkanes cyclopentane 108 134
Conformations of cycloalkanes medium and large rings 129
Conformations of ethers 667
Conformations peptides and proteins 1126Ч1128 1143Ч1145
Conformations s-cis and s-trans 401Ч402 417
Conformer 105 (see also УConformationФ)
Coniine 924
Conjugate acids and bases 33Ч38 368Ч370 593 763 797 919Ч920
Conjugate addition see also УMichael reactionФ
Conjugate addition of bromine to 1,3-butadiene 407
Conjugate addition of hydrogen bromide to 1,3-butadiene 407
Conjugate addition to  -unsaturated aldehydes and ketones 777Ч780 783Ч784 901Ч902 907 -unsaturated aldehydes and ketones 777Ч780 783Ч784 901Ч902 907
Conjugation energy 399Ч400
Conjugation in  -unsaturated aldehydes and ketones 775-777 -unsaturated aldehydes and ketones 775-777
Conjugation in alkenylbenzenes 447Ч448
Conjugation in allylic systems 391Ч398 405Ч407 416
Conjugation in benzylic carbocations 445
Conjugation in benzylic free radicals 441
Conjugation in dienes 398Ч402 565Ч566 conjugatedФ)
Connectivity see УConstitutionФ
Constitution 20 21 48
Constitutional isomers 23 48 192 315
Constitutional isomers of alkanes, number of 69 table
Coordination polymerization 271 408 610 612Ч614 617
Copolymer 408
Copper (I) salts in preparation of lithium dialkylcuprate 602Ч603 615
Copper (I) salts reactions with aryl diazonium ions 946 948 961 973
Corey, Elias J. 598 895
Corey, Robert B. 1143
Correlated spectroscopy COSY 556Ч559 577
Correlated spectroscopy HETCOR 558Ч559 577
Corticosteroids (cortisol and cortisone) 1098 1103
COSY see Correlated spectroscopy
Couper, Archibald S. 3
Coupling constant (J) 538 541 543Ч544
Coupling constant (J)dihedral angle dependence 580
covalent bond 12Ч13 47
COX-1and COX-2 see УCyclooxygenaseФ
Cracking, in petroleum refining 79
Crafts, James M. 481
Crick, Francis H. C. 1166Ч1170 1172
Critical micelle concentration 800
Crown ethers 668Ч671 692
Crown ethers electrostatic potential map 665 669
Cubane 140
Cumene 269 1023
Cumulated diene see УAllenes DienesФ
Cuprates see УLithium diorganocupratesФ
Curl, Robert F. 436
Curved arrows 34
Curved arrows and resonance structures 25 392
Curved arrows fishhook 169
Cyanide ion as nucleophile 327 328 338 347Ч348 349 352 777Ч779
Cyanide ion basicity of 349 777
Cyanide ion in formation of cyanohydrins 717Ч720
Cyanohydrins and carbohydrate chain extension 1055Ч1056 1063
Cyanohydrins hydrolysis of 809
Cyanohydrins naturally occurring 720
Cyanohydrins preparation of 717Ч720 743 867
Cyclic AMP 1161Ч1162
| Cycloaddition 409
Cycloaddition molecular orbital treatment of 414Ч415
Cycloalkanes 77Ч79 114Ч129 134Ч136
Cycloalkanes angle strain in 113 114Ч116
Cycloalkanes bicyclic and spirocyclic 129Ч131 136
Cycloalkanes conformations of 114Ч129 134Ч136
Cycloalkanes heats of combustion 113 table
Cycloalkanes nomenclature of 77Ч79
Cycloalkanes sources of 79Ч80
Cycloalkenes 190 200Ч201
Cycloalkenes nomenclature of 190
Cycloalkenes stereoisomeric 200Ч201 213
Cycloalkynes 365 368
Cycloamylose 1050
Cyclobutadiene 449Ч451 467
Cyclobutane angle strain in 113 115
Cyclobutane chlorination of 175
Cyclobutane conformations of 115
Cyclobutane heat of combustion of 113
Cyclobutyl chloride 175
Cyclodecane 113 180
Cyclodecyl bromide 213
Cyclodecyl chloride 180
Cyclodextrins 1049
Cycloheptatriene 457
Cycloheptatrienide anion 459
Cycloheptatrienyl cation 456Ч457 467 571
Cyclohexadienone-phenol rearrangement 1022
Cyclohexadienyl anion intermediate in nucleophilic aromatic substitution 977Ч981 987
Cyclohexadienyl cation intermediate in electrophilic aromatic substitution 474Ч476 478 479 481 482 485 489Ч494 496Ч497 501 506Ч507 509 980
Cyclohexane 77 80
Cyclohexane bond angles in 116
Cyclohexane conformational analysis of 116Ч120 134Ч135
Cyclohexane conformational analysis of disubstituted derivatives 125Ч129 135Ч136 305
Cyclohexane conformational analysis of monosubstituted derivatives 120Ч124 135
Cyclohexane drawing chair conformation of 118
Cyclohexane heat of combustion 113
Cyclohexane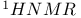 spectrum of 545 spectrum of 545
Cyclohexanol infrared spectrum 651 652
Cyclohexanol preparation of 247
Cyclohexanol reactions of dehydration 203
Cyclohexanol reactions of oxidation 642
Cyclohexanol reactions of with hydrogen bromide 152
Cyclohexanone  chlorination of 757 chlorination of 757
Cyclohexanone and ethylene glycol, cyclic acetal from 722
Cyclohexanone preparation of 642
Cyclohexanone reaction of reductive amination of 934
Cyclohexanone reaction of with ethylmagnesium bromide 713
Cyclohexanone reaction of with isobutylamine 673
Cyclohexanone reaction of with methylenetriphenylphosphorane 730
Cyclohexanone reaction of with morpholine 744
Cyclohexanone reaction of with pyrrolidine 936
Cyclohexanone reaction of with sodium acetylide 597
Cyclohexene derivatives of, preparation by Dieis Ч Alder reaction 409Ч415
Cyclohexene preparation of dehydration of cyclohexanol 203
Cyclohexene preparation of dehydrohalogenation 212
Cyclohexene preparation of reactions of alkylation of benzene with 483
Cyclohexene preparation of reactions of epoxidation 684
Cyclohexene preparation of reactions of hydroxylation 635 684
Cyclohexene preparation of reactions of trans stereoisomer 201
Cyclohexene preparation of reactions of with dibromocarbene 607
Cyclohexene preparation of reactions of with N-bromosuccinimide 397
Cyclohexene preparation of reactions of with sulfuric acid 247
Cyclohexyl chloride see also УChlorocyclohexaneФ
Cyclohexyl chloride  -elimination of 212 -elimination of 212
Cyclohexyl chloride Grignard reagent from 591 596
Cyclohexylamine 914
Cyclohexylamine basicity of 920
Cyclohexylamine preparation of 934
Cyclohexylamine reductive amination by 957
Cyclononyne 365
Cyclooctane 113
Cyclooctatetraene 449Ч451 452Ч453 465 467
Cyclooctatetraene dianion 459
Cyclooctene addition of chlorine to 256
Cyclooctene epoxidation of 261
Cyclooctene trans stereoisomer 201
Cyclooctyne 365
Cyclooxygenase 1080Ч1083
Cyclopentadiene acidity of 458Ч459
Cyclopentadiene Dieis Ч Alder reactions of 411
Cyclopentadiene reaction with hydrogen chloride 405
Cyclopentadienide anion 458 467
Cyclopentane 80
Cyclopentane conformations of 115 134
Cyclopentane heat of combustion 113
Cyclopentanol nitrate ester 656
Cyclopentanol preparation of 628
Cyclopentanol reaction with phosphorus tribromide 166
Cyclopentanone Baeyer Ч Villiger oxidation of 749
Cyclopentanone enamine of 728
Cyclopentanone enol content of 782
Cyclopentanone hydrogen-deuterium exchange in 768
Cyclopentanone hydrogenation of 628
Cyclopentanone reaction with methylmagnesium chloride 596
Cyclopentene bromine addition to 256
Cyclopentene halohydrins of 259Ч260
Cyclopentyl bromide 166 510
Cyclopentyl cyanide 328
Cyclopentylmethanol 626 636
Cyclopropane 1,1-dihalo 607
Cyclopropane heat of combustion of 113
Cyclopropane preparation of 604Ч606 615
Cyclopropane structure of 114
Cyclopropane torsional strain in 114
Cyclopropane(s) 77
Cyclopropanecarboxylic acid 632
Cyclopropanes angle strain and bonding in 113 114
Cyclopropanes cis and trans-l,2-dimethyl 124Ч125
Cyclopropene 200
Cyclopropenyl cation 459
Cyclopropyllithium 616
Cytidine 1159
Cytosine 1157 1166 1168
D-Alloisoleucine 1116
D-Allose 1031
D-Altrose 1031
D-Apiose 1043 1065
D-Arabinitol 1063
D-Arabinose 1031 1060Ч1061 1063
D-Erythrose 1029
D-Erythrose furanose forms 1033Ч1034
D-Fructose 1027 1041 1057
D-Fructose 6-phosphate 1057Ч1058
D-Galactal 1046
D-Galactitol 1052 1054
D-Galactose 1031
D-Galactose natural occurrence 1032
D-Galactose pyranose form 1038Ч1039
D-Galactose reduction of 1052
D-Glucose 140 625 1027 1032
D-Glucose 6-phosphate 1057 1161
D-Glucose conversion to D-fructose 1051 1057
D-Glucose electrostatic potential map 1026
D-Glucose epimerization of 1056Ч1057
D-Glucose Fischer determination of structure 1052 1068
D-Glucose hydrogenation of 658
D-Glucose metabolism 1069
D-Glucose methyl glycosides 1045Ч1046
D-Glucose mutarotation of 1040
D-Glucose natural occurrence 1032
D-Glucose oxidation of 1055
D-Glucose pyranose form 1037Ч1038
D-Glucuronic acid 1055
D-Glyceraldehyde 3-phosphate 1058
D-Glyceraldehyde Fischer projection formula 1028
D-Gulose 1031
D-Idose 1031
D-Lyxose 1031
D-Mannose 1031
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



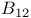
 and
and 
 -unsaturated aldehydes and ketones
-unsaturated aldehydes and ketones 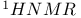 spectrum of
spectrum of