|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
Nomenclature IUPAC of nucleosides 1160
Nomenclature IUPAC of organometallic compounds 588 614
Nomenclature IUPAC of sulfides 666
Nomenclature IUPAC of thiols 648
Nomenclature stereochemical notation cis and trans 124
Nomenclature stereochemical notation D-L 293 1027Ч1032 1061
Nomenclature stereochemical notation E-Z 193Ч195 220
Nomenclature stereochemical notation erythro and threo 301Ч302
Nomenclature stereochemical notation R-S 290Ч293
Nomenclature substitutive 144Ч145 178
Nomex 868
Nonsteroidal antiinflammatory drugs 1083
Norepinephrine 687Ч688 1126
Norethindrone 1100
NSAIDs see УNonsteroidal antiinflammatory drugsФ
Nuclear magnetic resonance spectra carbon 1-chloropentane 548
Nuclear magnetic resonance spectra carbon 1-phenyl-1-pentanone 553-554
Nuclear magnetic resonance spectra carbon 3-heptanone 740
Nuclear magnetic resonance spectra carbon m-cresol 551Ч552
Nuclear magnetic resonance spectra carbon methanol 953
Nuclear magnetic resonance spectra carbon methylamine 953
Nuclear magnetic resonance spectra proton 1,1-dichloroethane 536
Nuclear magnetic resonance spectra proton 1-chloropentane 548
Nuclear magnetic resonance spectra proton 2,3,4-trichloroanisole 542
Nuclear magnetic resonance spectra proton 2-butanone 739
Nuclear magnetic resonance spectra proton 2-methylpropanal 739
Nuclear magnetic resonance spectra proton 2-phenylethanol 653
Nuclear magnetic resonance spectra proton 4-methylbenzyl alcohol 952Ч954
Nuclear magnetic resonance spectra proton 4-methylbenzylamine 952Ч954
Nuclear magnetic resonance spectra proton 4-phenylbutanoic acid 820
Nuclear magnetic resonance spectra proton benzyl alcohol 545
Nuclear magnetic resonance spectra proton chloroform 525
Nuclear magnetic resonance spectra proton dipropyl ether 689
Nuclear magnetic resonance spectra proton ethyl acetate 872 873
Nuclear magnetic resonance spectra proton ethyl bromide 538Ч539
Nuclear magnetic resonance spectra proton isopropyl chloride 540
Nuclear magnetic resonance spectra proton m-nitrostyrene 543
Nuclear magnetic resonance spectra proton methoxyacetonitrile 532Ч533
Nuclear magnetic resonance spectra proton methyl propanoate 872 873
Nuclear magnetic resonance spectra proton p-cresol 1015
Nuclear magnetic resonance spectroscopy and magnetic field strength 522Ч523
Nuclear magnetic resonance spectroscopy carbon 547Ч559 576Ч577
Nuclear magnetic resonance spectroscopy carbon alcohols 652
Nuclear magnetic resonance spectroscopy carbon aldehydes and ketones 738 740
Nuclear magnetic resonance spectroscopy carbon amines 953
Nuclear magnetic resonance spectroscopy carbon carboxylic acid derivatives 872Ч873
Nuclear magnetic resonance spectroscopy carbon carboxylic acids 819Ч820
Nuclear magnetic resonance spectroscopy carbon chemical shifts 549Ч551
Nuclear magnetic resonance spectroscopy carbon ethers 690Ч691
Nuclear magnetic resonance spectroscopy carbon in biosynthetic studies 1092
Nuclear magnetic resonance spectroscopy carbon thiols 652
Nuclear magnetic resonance spectroscopy proton 522Ч545 575Ч576
Nuclear magnetic resonance spectroscopy proton alcohols 544Ч545 576 651Ч652 653 655
Nuclear magnetic resonance spectroscopy proton aldehydes and ketones 738Ч741
Nuclear magnetic resonance spectroscopy proton amines 952Ч953
Nuclear magnetic resonance spectroscopy proton and conformations 544Ч545 576
Nuclear magnetic resonance spectroscopy proton carboxylic acid derivatives 872 873
Nuclear magnetic resonance spectroscopy proton carboxylic acids 819Ч820
Nuclear magnetic resonance spectroscopy proton chemical shift 525Ч532 575
Nuclear magnetic resonance spectroscopy proton ethers and epoxides 689Ч692
Nuclear magnetic resonance spectroscopy proton interpretation 532Ч535 576
Nuclear magnetic resonance spectroscopy proton nuclear shielding 525Ч526
Nuclear magnetic resonance spectroscopy proton phenols 1014Ч1015
Nuclear magnetic resonance spectroscopy proton spin-spin splitting 535Ч544 576
Nuclear magnetic resonance spectroscopy proton thiols 652
Nuclear magnetic resonance spectroscopy two dimensional (2D NMR) 556Ч559 577
Nuclear spin states 522Ч523
Nucleic acids 1165Ч1166 (see also УDeoxyribonucleic acidФ; УRibonucleic acidФ)
Nucleophiles 47 157Ч158 159Ч160 163Ч165 181 327Ч329
Nucleophiles relative reactivity 336Ч338
Nucleophiles solvation and reactivity 338 346Ч347
Nucleophilic acyl substitution 830Ч885
Nucleophilic acyl substitution defined 830Ч831
Nucleophilic acyl substitution general mechanism 836Ч838 874Ч875
Nucleophilic acyl substitution of acyl chlorides 838Ч841 875
Nucleophilic acyl substitution of amides 862Ч867 877
Nucleophilic acyl substitution of carboxylic acid anhydrides 841Ч842 875
Nucleophilic acyl substitution of esters 848Ч858 876
Nucleophilic acyl substitution of thioesters 858Ч859 876
Nucleophilic addition to  -unsaturated aldehydes and ketones 777Ч779 784 901Ч902 907 -unsaturated aldehydes and ketones 777Ч779 784 901Ч902 907
Nucleophilic addition to aldehydes and ketones 712Ч735 742Ч744
Nucleophilic alkyl substitution (see also У 2$"/> SNФ) 2$"/> SNФ)
Nucleophilic alkyl substitution  -halo carboxylic acids 816 -halo carboxylic acids 816
Nucleophilic alkyl substitution alcohols 153Ч166
Nucleophilic alkyl substitution alkyl halides 326Ч350 733 808 867 894Ч900
Nucleophilic alkyl substitution alkyl p-toluenesulfonates 350Ч353 357
Nucleophilic alkyl substitution allylic halides 391Ч394 416 650 895
Nucleophilic alkyl substitution benzylic halides 444Ч446
Nucleophilic alkyl substitution crown ether catalysis of 671
Nucleophilic alkyl substitution enzyme-catalyzed 339
Nucleophilic alkyl substitution epoxides 678Ч684
Nucleophilic alkyl substitution phase-transfer catalysis of 923 926
Nucleophilic aryl substitution 975Ч985 986Ч987 1000 1009
Nucleosides 1158Ч1160
Nucleosomes 1171
nucleotides 1160Ч1162 1164Ч1166 1187
Nylon 4 868
O-Acetylsalicylic acid see УAspirinФ
o-Cresol 1003
o-Hydroxybenzoic acid 792 (see also УSalicylic acidФ)
o-Nitroaniline, diazotization of 961
o-Nitrophenol acidity of 998
o-Nitrophenol intramolecular hydrogen bonding 996
o-Nitrophenol reaction with acetic anhydride 1005 1017
o-Nitrophenol reaction with butyl bromide 1018
o-Toluidine 948
o-Xylene 432Ч433
o-Xylene Birch reduction of 464Ч465
Octadecanoic acid 792
Octane number of gasoline 80
Octane, relative stability of isomers 85Ч86
Octet rule 13 47
Off-resonance decoupling 553
Oil of wintergreen see УMethyl salicylateФ
Oils see УFatsФ
Olah, George A. 83
Olefin 189 (see also УAlkenesФ)
Oleic acid 193 792 1073
Oligonucleotide 1164
Oligosaccharide 1027
Opsin 729
Optical activity 287Ч289 316
Optical activity and chemical reactions 297Ч300 309 316 331Ч333 342Ч344 353 354 768Ч769
Optical purity 288
Optical resolution see УResolutionТ
Orbital hybridization 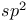 in alkadienes 400Ч402 in alkadienes 400Ч402
Orbital hybridization 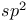 in aniline 917Ч918 in aniline 917Ч918
Orbital hybridization 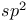 in benzene 430 in benzene 430
Orbital hybridization 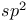 in carbenes 607 in carbenes 607
Orbital hybridization 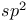 in carbocations 156 161 181 in carbocations 156 161 181
Orbital hybridization 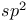 in ethylene and alkenes 89Ч92 95 190Ч192 220 in ethylene and alkenes 89Ч92 95 190Ч192 220
Orbital hybridization 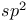 in formaldehyde 706Ч707 in formaldehyde 706Ч707
Orbital hybridization 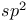 in free radicals 168 181 in free radicals 168 181
Orbital hybridization 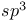 in alkyl halides 146 in alkyl halides 146
Orbital hybridization 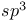 in ethane 67 95 in ethane 67 95
Orbital hybridization 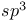 in methane 63Ч65 95 in methane 63Ч65 95
Orbital hybridization 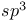 in methanol 146 in methanol 146
Orbital hybridization 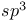 in methylamine 916 917 in methylamine 916 917
Orbital hybridization model for bonding 63Ч65 95
Orbital hybridization sp in acetylene and alkynes 92Ч94 95 365Ч367 382
Orbital hybridization sp in allenes 402Ч404
Orbital symmetry, and Diets Ч Alder reaction 414Ч415
Orbitals atomic 7Ч10 47
Orbitals hybrid orbitals 63Ч65 89Ч92 92Ч94 95
Organic chemistry definition 1
Organic chemistry historical background of 1Ч6
Organic Structure Elucidation 555
Organoboranes 250Ч251 252Ч254
Organocopper compounds see УLithium diorganocupratesФ
Organolithium reagents basicity of 592Ч594 614
| Organolithium reagents preparation of 589Ч590 615
Organolithium reagents reaction of with aldehydes and ketones 594Ч596 616 617 626
Organolithium reagents reaction of with epoxides 632Ч633
Organolithium reagents reaction of with nitriles 872
Organomagnesium compounds see УGrignard reagentsФ
Organometallic compounds 587Ч622 (see also УGrignard reagentsФ; УLithium diorganocupratesФ; УOrganolithium reagentsФ; УOrganozinc compoundsФ)
Organozinc compounds 604Ч606 615
Ortho (o), disubstituted organic compounds 432Ч433
Ortho-para directing groups 488Ч492 494Ч497 495 500Ч501
Osmium tetraoxide 634Ч635 654
Oxalic acid 54 804
Oxane 637 666
Oxaphosphetane 732
Oxazole 461
Oxidation (see also УEpoxidationФ; УHydroxylation of alkenesФ; УOzonolysisФ)
Oxidation biological 285 435 444 645Ч647
Oxidation of alcohols 641Ч648 657 709Ч711 807
Oxidation of aldehydes 736 745 807
Oxidation of alkylbenzenes 443Ч444 466 806 807
Oxidation of carbohydrates 1053Ч1055 1063
Oxidation of ketones 736Ч738 745
Oxidation of phenols 1012Ч1013 1018
Oxidation of vicinal diols 647Ч648 655
Oxidation-reduction in organic chemistry 87Ч89 98
Oximes 727
Oxkane 666 (see also УEthylene oxideФ)
Oxo process see УHydroformylationФ
Oxolane 666 (see also УTetrahydrofuranФ)
Oxonium ion 34 (see also УHydronium ionФ)
Oxygen biological storage and transport of 1148 1150
Oxygen isotopic labels 810 852 854Ч855
Oxytocin 1129
Ozone, bonding in 24Ч25 262
Ozonide 263
Ozonolysis of alkenes 262Ч264 274 710
Ozonolysis of alkynes 381Ч382
p-(Trifluoromethyl)aniline 921
p-Aminobenzoic acid 942 951
p-Chloronitrobenzene, nucleophilic substitution in 975Ч977
p-Chloronitrobenzene, nucleophilic substitution in electrostatic potential map 971
p-Cresol, 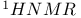 spectrum 1014Ч1015 spectrum 1014Ч1015
p-Cresol, acidity of 998
p-Cresol, carboxylation 1008
p-Cresol, infrared spectrum 1014
p-Cresol, nitration of 1003
p-Cresol, preparation of 1000
p-Fluoronitrobenzene, nucleophilic aromatic substitution in 976Ч979 1009
p-Fluorophenol, O-acylation 1005
p-Nitroaniline basicity of 921
p-Nitroaniline bromination of 959
p-Nitroaniline preparation of 941Ч942
p-Nitrophenol acidity of 998
p-Nitrophenol esters of, in peptide bond formation 1139 1141
p-Toluenesulfonic acid as acid catalyst 722
p-Toluenesulfonic acid, acidity of 351
p-Toluenesulfonic acid, esters as substrates in nucleophilic aliphatic substitution 350Ч353 357
p-Toluenesulfonic acid, esters preparation of 351 357 636
p-Toluenesulfonic acid, nucleophilic aromatic substitution in 1000
p-Toluenesulfonyl chloride, reaction with alcohols 351 357 636
p-Xylene 432Ч433
p-Xylene Friedel Ч Crafts acylation of 502
p-Xylene oxidation of 806
Palladium hydrogenation catalyst 230 231 627Ч628
Palladium Lindlar 374Ч375 384
Palmitic acid 1073
Papain 1130
Para (p), disubstituted organic compounds 432Ч433
Paraffin hydrocarbon 83 (see also УAlkanesФ)
Partial rate factors 490Ч491 502 517
Pasteur, Louis 310
Pauli exclusion principle 8Ч9
Pauling, Linus 3 15
Pauling, Linus and orbital hybridization 64
Pauling, Linus and peptide structure 1143Ч1145
Pauling, Linus electronegativity scale 15
PCBs see УPolychlorinated biphenylsФ
PCC see УPyridinium chlorochromateФ
PCR see УPolymerase chain reactionФ
PDC see УPyridinium dichromateФ
Pedersen, Charles J. 669
Penicillin G 861Ч862
pentaacetate 1058
Pentane 71 82
Pentane conformation of 110 112
Pentanenitrile hydrogenation of 932
Pentanenitrile preparation of 923 926
Pentobarbital 900
Pentothal sodium 901
Pentyl azide 927
Pepsin 1130
Peptide bond 1109 1126
Peptide bond geometry of 1127Ч1128
Peptide bond preparation of 1139Ч1143
Peptides 1126Ч1147 (see also УProteinsФ)
Peptides amino acid analysis 1130
Peptides classification of 1109
Peptides end-group analysis of 1131Ч1135
Peptides hydrolysis of 1130
Peptides structure of 1109 1126Ч1129
Peptides synthesis of 1135Ч1143
Pericyclic reactions 409 1012
Periodic acid cleavage of carbohydrates 1059Ч1061 1064
Periodic acid cleavage of vicihal diols 647Ч648 655
Perkin, William Henry 4
Peroxide effect 243
Peroxides by oxidation of ethers 674
Peroxides initiators of free-radical reactions 242Ч244 442-443
Peroxides intermediates in icosanoid biosynthesis 1081Ч1082
Peroxyacetic acid 796
Peroxyacetic acid epoxidation of alkenes 261Ч262 273 676 693
Peroxybenzoic acid 736Ч738
Perutz, Max F. 1146
Petrochemicals 5 189
Petroleum 79Ч80
Pharmacology 952
Phase-transfer catalysis 923 926 956
Phenacetin 1021
Phenanthrene 434Ч435
Phenobarbital 901
Phenol(s) 993Ч1025
Phenol,  of 36 of 36
Phenol, acidity of 45 996Ч999 1016
Phenol, electrostatic potential maps 993 996
Phenol, formation of, in Claisen rearrangement 1011 1018
Phenol, hydrogen bonding 995Ч996
Phenol, naturally occurring 1001Ч1002
Phenol, nomenclature of 432 993Ч994
Phenol, physical properties 995Ч996
Phenol, preparation from aryl diazonium salts 946 960 1001 1016
Phenol, preparation from benzenesulfonic acid 1000
Phenol, preparation from chlorobenzene 975 1000
Phenol, preparation from cumene 1000
Phenol, reactions of azo coupling 1004
Phenol, reactions of bromination 1002Ч1003
Phenol, reactions of carboxylation 1006Ч1008 1017
Phenol, reactions of electrophilic aromatic substitution 494 1002Ч1004
Phenol, reactions of esterification 1005 1006 1017
Phenol, reactions of Friedel Ч Crafts acylation 1004 1005
Phenol, reactions of Friedel Ч Crafts alkylation 1003
Phenol, reactions of Kolbe Ч Schmitt reaction 1006Ч1008 1017
Phenol, reactions of nitration 494 1003
Phenol, reactions of nitrosation 1003
Phenol, reactions of O-alkylation 1008 1018
Phenol, reactions of oxidation 1012Ч1014 1018
Phenol, reactions of sulfonation 1003
Phenol, resonance in 995
Phenol, spectroscopic analysis 1014Ч1015
Phenol, structure and bonding 994Ч995
Phenyl benzoate, Fries rearrangement of 1006
Phenyl group 434
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 -unsaturated aldehydes and ketones
-unsaturated aldehydes and ketones 2$"/>
2$"/> -halo carboxylic acids
-halo carboxylic acids 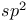 in alkadienes
in alkadienes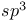 in alkyl halides
in alkyl halides 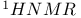 spectrum
spectrum of
of