|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
Phenylacetic acid  -halogenation 816 -halogenation 816
Phenylacetic acid preparation of 808
Phenylalanylglycine, synthesis of 1136Ч1139 1141
Phenylbutazone 911
Phenylhydrazine, reaction of, with aldehydes and ketones 727
Phenylisothiocy anate 1134
Phenylketonuria (PKU disease) 1125
Phenyllithium 590
Phenylmagnesium bromide carboxylation of 808
Phenylmagnesium bromide preparation of 591 974
Phenylmagnesium bromide, reaction of with 1,2-epoxypropane 681
Phenylmagnesium bromide, reaction of with 2-butanone 600
Phenylmagnesium bromide, reaction of with ethyl benzoate 616
Phenylmagnesium bromide, reaction of with methanol 592
Phenylpyruvic acid 1125
Phenylthiohydantoin 1134Ч1135
Pheromone, aggregating of cockroach 68 71
Pheromone, aggregating of European elm bark beetle 661
Pheromone, alarm pheromone of ant 709
Pheromone, alarm pheromone of bees 709
Pheromone, sex attractant of boll worm moth 881
Pheromone, sex attractant of codling moth 224
Pheromone, sex attractant of female gypsy moth 261
Pheromone, sex attractant of female house fly 194 388
Pheromone, sex attractant of female Japanese beetle 845
Pheromone, sex attractant of female tiger moth 101
Pheromone, sex attractant of female winter moth 750
Pheromone, sex attractant of greater wax moth 709
Pheromone, sex attractant of honeybee 224
Pheromone, sex attractant of male Oriental fruit moth 845
Pheromone, sex attractant of Mediterranean fruit fly 224
Pheromone, sex attractant of Western pine beetle 748
Phosphatidic acid 1077
Phosphatidylcholine 1078Ч1079
Phosphines as nucleophiles 733
Phosphines optically active 314
Phosphoglucose isomerase 1057
Phosphoglycerides 1078
Phospholipid bilayer 1078Ч1079
Phospholipids 1077Ч1079
Phosphoric acid, catalyst for alcohol dehydration 203 204 208
Phosphoric acid, esters of 641 1160Ч1161
Phosphorous acid esters 641
Phosphorus pentoxide 869
Phosphorus tribromide, reaction with alcohols 165Ч166 180
Phosphorus ylides see УYlidesФ
Phosphorylation 1161 1163
photochemical chlorination of 185
Photochemical initiation of addition of hydrogen bromide to alkenes 244 274
Photochemical initiation of free-radical reactions 175 244 274
Photon 520
Photosynthesis 1032 1069
Phthalhydrazide 930
Phthalic acid see У1 acidФ
Phthalic anhydride 841 843 862
Phthalimide 862
Phthalimide potassium salt of, in Gabriel synthesis 929Ч931 956
Physical properties see УSpecific compound classФ
Physostigmine 963
Phytane 73
Piperidine 132 839 989
Piperidine basicity 922
Piperidine in reductive amination 935
pKb 37Ч38 (see also УBasicityФ)
PKU disease see УPhenylketonuriaФ
Planck, Max 520
PlanckТs constant 520
Plane of symmetry 286Ч287
Plane of symmetry cis-1,2-dibromocyclopropane 304
Plane of symmetry in meso-2,3-butanediol 303Ч304
Plane-polarized light 287Ч289
Platinum, hydrogenation catalyst 230 231 271Ч272 428 627Ч628
Pleated  -sheet 1144 -sheet 1144
Poison ivy, allergens in 1022
Polar covalent bonds see УBonds polar
Polar solvents 329 345Ч348
Polarimeter 287Ч289
Polarizability 149Ч150
Poly(vinyl alcohol) 883
Poly(vinyl chloride) 190 269 270
Polyamides 868Ч869
Polyamines 925
Polychlorinated biphenyls 992
Polycyclic hydrocarbons aliphatic 129Ч131
Polycyclic hydrocarbons aromatic 434Ч435 506Ч507
Polycyclic hydrocarbons aromatic and cancer 435
Polyesters 868
Polyethers 668Ч671
Polyethylene 267Ч268 269 270 271 610 612Ч614 617
Polyisoprene 270 408
Polymerase chain reaction (PCR) 1183Ч1186
Polymerization cationic 266
Polymerization condensation polymers 868Ч869
Polymerization coordination 271 312Ч314 408 610 612Ч614 617
Polymerization free-radical 267Ч268
Polymers 266Ч270
Polymers of dienes 408
Polymers polyamides 868Ч869
Polymers polyesters 868
Polymers stereoregular 312Ч314 317Ч318 610 612Ч614 617
Polymers vinyl 270
Polynucleotides 1164 (see also УNucleic acidsФ)
Polypeptide 1109 (see also УPeptidesФ; УProteinsФ)
Polypropylene 269 270 271 312Ч314 612
Polysaccharide 1027 1048Ч1050 1062
polystyrene 269 270 449
Polyurethanes 269
Poreda, Robert J. 437
Porphyrin 1147
Postage stamps 1Ч5
Potassiophthalimide see УPhthalimideФ
Potassium dichromate (see also УChromic acid oxidationФ)
Potassium dichromate of alcohols 641 643
Potassium dichromate of aldehydes 736 807
Potassium permanganate oxidation of alcohols 641 807
Potassium permanganate oxidation of aldehydes 807
Potassium permanganate oxidation of alkylbenzenes 443 466 807
Potassium tert-butoxide base in elimination reactions 212 373 606 607
potential energy 85
Potential energy and heat of combustion 85Ч87 124Ч125 197
Potential energy and heat of hydrogenation 232
Potential energy diagrams 155Ч159
Potential energy diagrams addition of hydrogen bromide to 1,3-butadiene 407
Potential energy diagrams and MarkovnikovТs rule 239
Potential energy diagrams bimolecular elimination (E2) 215
Potential energy diagrams bimolecular nucleophilic substitution 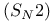 333 333
Potential energy diagrams branched versus unbranched alkanes 85
Potential energy diagrams carbocation formation 157
Potential energy diagrams carbocation rearrangement 210
Potential energy diagrams conformations of 1,3-butadiene 401Ч402
Potential energy diagrams conformations of butane 110
Potential energy diagrams conformations of cyclohexane 120
Potential energy diagrams conformations of ethane 107
Potential energy diagrams electrophilic aromatic substitution 475 491 494
Potential energy diagrams hydration of aldehydes and ketones 717
Potential energy diagrams proton transfer 155
Potential energy diagrams reaction of tert-butyl alcohol with hydrogen chloride 159
Potential energy diagrams unimolecular nucleophilic substitution (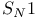 ) 159 341 ) 159 341
Pott, Sir Percivall 435
Prelog, Vladimir 194
Priestley, Joseph 408
Primary carbon 74
principal quantum number 8
Pristane 183
Prochiral 297 299
Progesterone 1100
Prontosil 951
Propagation step 172Ч173 175 182 243Ч244 442
Propanal 707
Propane attractive forces in 148
| Propane bond dissociation energies in 169 170
Propane chemical shifts carbon 549
Propane chemical shifts proton 527
Propane conformational analysis of 110
Propane dehydrogenation of 189 202
Propane dipole moment of 148 918
Propane in natural gas 63
Propene 187Ч188
Propene as industrial chemical 269
Propene, addition of sulfuric acid to 246
Propene, allylic chlorination of 396
Propene, bond dissociation energy of 395 439
Propene, bond distances in 191 367 400
Propene, dipole moment of 196
Propene, epoxidation of 297
Propene, heat of hydrogenation of 233 399Ч400
Propene, hydration rate of 249
Propene, polymerization of 271 312Ч314 612
Propene, structure 191
Propyl group 74
Propyl radical 169
Propylene 187 (see also УPropeneФ)
Propylene glycol 634
Propylene oxide 269 (see also У1
Prostacyclins 1082
Prostaglandins 144 791 1080Ч1084
Prosthetic groups see УCoenzymesФ
Protease inhibitors 1180
Protecting groups acetals as 723Ч724
Protecting groups for amino acids 1137Ч1139
Protecting groups for arylamines 940Ч942
Protein Data Bank 1146
Proteins amino acid analysis of 1130
Proteins biosynthesis of 1178 1179
Proteins glycoproteins 1050
Proteins, hydrolysis of 1130
Proteins, structure of primary 1129Ч1135 1151
Proteins, structure of quaternary 1148 1150 1152
Proteins, structure of secondary 1143Ч1145 1152
Proteins, structure of tertiary 1145Ч1148 1152
Proteins, synthesis of 1135Ч1143
Protic solvents 346Ч347
Proton magnetic resonance spectra see УNuclear magnetic resonance spectraФ
Proton magnetic resonance spectroscopy see УNuclear magnetic resonance spectroscopyФ
Proton-transfer reactions see УAcid-base reactionsФ
Pseudoionone 1107
Purcell, Edward 522
Purines 461 1155Ч1158 1186
Purines, hydrogen bonding in 1166 1168
Purines, nucleosides of 1158Ч1160 1187
Purines, nucleotides of 1160Ч1162 1187
Purines, polynucleotides of 1164Ч1166 1188
Putrescine 925
Pyramidal inversion 314
Pyranose forms of carbohydrates 1036Ч1039 1062
Pyrethrins 1105
Pyridine 460
Pyridine,  of conjugate acid 36 38 of conjugate acid 36 38
Pyridine, acylation catalyst 639 839 841
Pyridine, basicity of 37Ч38 922
Pyridine, bonding in 462Ч463
Pyridine, electrophilic aromatic substitution in 507Ч508
Pyridinium chlorochromate (PCC) 642 657 710
Pyridinium dichromate (PDC) 642 657 710
Pyridoxal phosphate 728
Pyrimidines 1155Ч1158 1186
Pyrimidines, hydrogen bonding in 1166 1168
Pyrimidines, nucleosides of 1158Ч1160 1187
Pyrimidines, nucleotides of 1160Ч1162 1187
Pyrimidines, polynucleotides of 1164Ч1166 1188
Pyrocatechol 994 1010
Pyrrole 460
Pyrrole, bonding in 462Ч463
Pyrrole, electrophilic aromatic substitution in 507Ч508
Pyrrolidine 132
Pyrrolidine, acetylation of 928
Pyrrolidine, enamine of 730 936
Pyruvic acid, acetyl coenzyme A from 1070
Pyruvic acid, biological reduction of 735
Pyruvic acid, biosynthesis of 647 1069
Pyruvic acid, conversion to L-alanine 1123Ч1125
Quantized energy states 521
Quantum 520
Quantum numbers 8
Quaternary ammonium salts 916
Quaternary ammonium salts as phase-transfer catalysts 923 926 956
Quaternary ammonium salts hydroxides, Hofmann elimination of 938Ч940 958
Quaternary ammonium salts, preparation of 928 937Ч938
Quaternary carbon 74
Quaternary struture of proteins 1148 1150
Quinine 924
Quinoline 460
Quinones 1012Ч1013 1018
R-S-notational system 290Ч293 316
Racemic mixture 288 297 316
Racemic mixture, resolution of 310Ч312 317
Racemization and chair-chair interconversion 305
Racemization in 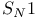 reactions 342Ч344 reactions 342Ч344
Racemization via enol 768Ч769
Radio waves 520
Random coils 1145
Rare gas see УNoble gas electron configurationФ
Rate constant 163
Rate of reaction (see also ЂSubstituent effectsФ)
Rate of reaction and carbocation stability 159Ч163 341Ч342
Rate of reaction, effect of catalyst on 231
Rate of reaction, effect of temperature on 108Ч109 163
Rate-determining step 159 853
Rearrangement allylic 394 406Ч407 416
Rearrangement Claisen rearrangement 1011Ч1012 1018
Rearrangement Fries rearrangement 1006
Rearrangement in  reactions 344Ч345 reactions 344Ч345
Rearrangement in alcohol dehydration 208Ч211 222Ч223
Rearrangement in Baeyer Ч Villiger oxidation 736Ч738 847
Rearrangement in electrophilic addition to alkenes 241Ч242
Rearrangement in Friedel Ч Crafts alkylation 482Ч483 511
Rearrangement in reactions of alcohols with hydrogen halides 355 357
Reducing sugar 1053
Reduction 87Ч89 (see also УHydrogenationФ; УHydrogenolysisФ)
Reduction Birch reduction 438Ч439 440 464Ч465
Reduction Clemmensen 486Ч488 505 713
Reduction metal-ammonia reduction of alkynes 376Ч377
Reduction of aldehydes and ketones 627Ч631 634 654 713
Reduction of amides 933 957
Reduction of aryl diazonium salts 948Ч950 961
Reduction of azides 931 957
Reduction of carbohydrates 1052Ч1053 1063
Reduction of carbonyl groups, agents for 654 table
Reduction of carboxylic acids 632 654 711 810
Reduction of esters 632 654
Reduction of imines 934Ч935
Reduction of nitriles 932 957
Reduction of nitro groups 932 957
Reduction Wolff Ч Kishner 487 713
Reductive amination 934Ч935 957
Refining of petroleum 79Ч80
Reforming, in petroleum refining 80
Regioselectivity addition of bromine to 1,3-butadiene 407
Regioselectivity addition of hydrogen halides to 1,3-butadiene 405-407
Regioselectivity allylic halogenation 396Ч398 416
Regioselectivity and MarkovnikovТs rule 238Ч241 272 273
Regioselectivity and regiospecificity 310
Regioselectivity and ZaitsevТs rule 204Ч205 221
Regioselectivity dehydration of alcohols 204 221 404 417 446
Regioselectivity dehydrohalogenation of alkyl halides 212Ч213 218 223 404 446
Regioselectivity electrophilic addition to alkenes 238Ч241 246 247Ч252 259Ч260 272 273
Regioselectivity electrophilic aromatic substitution 488Ч508
Regioselectivity epoxide ring opening 678Ч684 694
Regioselectivity Hofmann elimination 938Ч940 958
Regioselectivity hydration of alkynes 379Ч381 385
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 -halogenation
-halogenation  -sheet
-sheet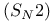
 )
)  of conjugate acid
of conjugate acid  reactions
reactions