|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
Arylamines basicity of 920Ч922
Arylamines in reductive amination 934
Arylamines nomenclature of 913-916
Arylamines preparation of 932
Arylamines reactions of acylation 940Ч942
Arylamines reactions of electrophilic aromatic substitution 497 939Ч942 959
Arylamines reactions of nitrosation 945Ч950
Arylamines structure and bonding 916Ч918
Ascaridole 1104
Ascorbic acid (vitamin C) 54 827 1034 1055
Aspartame 1051Ч1052 1125
Aspirin 54
Aspirin inhibition of prostaglandin biosynthesis by 1083
Aspirin preparation of 1006Ч1008
Asymmetric center see УChkality centerФ
Atactic polymers 313 610
Atomic number 7
Atomic number and the sequence rule 193
ATP see УAdenosine triphosphateФ
Avery, Oswald 1166
Axial bonds in cyclohexane 117Ч120 135
Azeotropic mixture 638 722
Azide ion 31 327 328 338 347 349 779 927
Azo coupling 950Ч951 1004
Azo dyes 951Ч952
AZT see УZidovudineФ
Baeyer strain theory 112Ч113
Baeyer Ч Villiger oxidation 736Ч738 745 847
Barbiturates 900Ч901
Barton, Sir Derek 116
Base pairs 1166Ч1168
Base peak 569
Bases, used in elimination reactions 211Ч213 372Ч373 383 606
Basicity and nucleophilicity 348Ч350
Basicity constant 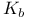 and and 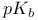 37Ч38 50 919 37Ч38 50 919
Basicity definition Arrhenius 33
Basicity definition Bronsted Ч Lowry 33
Basicity definition Lewis 45
Basicity of amines 919Ч923 955Ч956
Basicity of Grignard reagents 592Ч594 598
Basicity of heterocyclic amines 922Ч923
Basicity of leaving groups 330 352 944
Basicity of organolithium compounds 592Ч594
Becker, Luann 437
Beeswax 71 80 1079
Bender, Myron 852 855
Bending vibrations in infrared spectroscopy 559Ч560
BenedictТs reagent 1053Ч1054 1063
Benzal chloride 442
Benzaldehyde 432
Benzaldehyde diethyl acetal of 720
Benzaldehyde preparation of 711
Benzaldehyde reactions of Claisen Ч Schmidt condensation 775 783
Benzaldehyde reactions of nitration 498 927
Benzaldehyde reactions of reductive amination 935
Benzaldehyde reactions of with methylamine 724Ч726 927
Benzaldehyde reactions of with vinyllithium 597
Benzenamine 914 (see also УAnilineФ)
benzene 58 424Ч431 463Ч464
Benzene,  of 37 of 37
Benzene, acidity of 593 621
Benzene, as industrial chemical 424
Benzene, Birch reduction of 438Ч439 440
Benzene, derivatives, nomenclature of 432Ч434
Benzene, electrophilic aromatic substitution in 475 table
Benzene, electrophilic aromatic substitution in bromination 475 480Ч481 504 505
Benzene, electrophilic aromatic substitution in chlorination 475 480
Benzene, electrophilic aromatic substitution in Friedel Ч Crafts acylation 475 484Ч488 504 505
Benzene, electrophilic aromatic substitution in Friedel Ч Crafts alkylation 475 481Ч483 510
Benzene, electrophilic aromatic substitution in nitration 475 477Ч478 505
Benzene, electrophilic aromatic substitution in sulfonation and disulfonation 475 478Ч480 500
Benzene, electrostatic potential map 423
Benzene, heat of hydrogenation 428Ч429
Benzene, isolation and discovery 424
Benzene, mass spectrum 568Ч569
Benzene, molecular orbitals 430Ч431 452Ч453
Benzene, nuclear shielding in 529
Benzene, stability of 428Ч429 463
Benzene, structure and bonding 424Ч428
Benzene, structure and bonding Kekule formulation 424Ч427 463
Benzene, structure and bonding orbital hybridization model 430
Benzene, structure and bonding resonance description 427Ч428
Benzenecarbaldehyde see УBenzaldehydeФ
Benzenecarboxylic acid see УBenzoic acidФ
Benzenediazonium chloride 946 1004
Benzenediols 994 (see also УHydroquinoneФ; УPyrocatecholФ; УResorcinolФ)
Benzenesulfonic acid preparation of 475 478Ч480
Benzenesulfonic acid reactions of 500 1000
Benzimidazole 461
Benzofuran 460
Benzoic acid 424 432 792
Benzoic acid acidity of 803
Benzoic acid by oxidation of toluene 444
Benzoic acid esterification of 638 810Ч813
Benzonitrile 832
Benzophenone 706
Benzothiophene 460
Benzotrichloride 442
Benzoyl chloride 500 839 840
Benzoyl peroxide 442
Benzo[a]pyrene 435
Benzyl alcohol 711
Benzyl alcohol 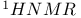 spectrum 545 spectrum 545
Benzyl bromide 434
Benzyl cation 438 445 571
Benzyl chloride, nucleophilic substitution in 672 784 808 841
Benzyl chloride, preparation of 442
Benzyl chloride, reaction of with lithium dimethylcuprate 617
Benzyl chloride, reaction of with magnesium 615
Benzyl chloride, reaction of with N-potassiophthalimide 930
Benzyl group 434
Benzyl radical 438 439 441Ч442
Benzylamine, preparation of 930
Benzylic 439
Benzylic halides, nucleophilic substitution in 444Ч446
Benzylic halogenation 439 441Ч443 466
Benzylic hydrogens 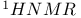 chemical shifts of 529 chemical shifts of 529
Benzyloxycarbonyl protecting group in peptide synthesis 1137Ч1139 1151
Benzyne as intermediate in nucleophilic aromatic substitution 981Ч985
Benzyne bonding in 982 984
Benzyne Dieis Ч Alder reactions of 985Ч986
Benzyne electrostatic potential map 984
Benzyne generation of 983 985Ч986 987
Berg, Paul 1181
Bergstrom, Sune 1084
Berthelot, Pierre Ч Eugene Marcellin 363
Berzelius, Jons Jacob 1Ч2 23
Bicarbonate 45 805
Bicarbonate  of 36 of 36
Bicyclic ring systems 130Ч131 136
Bicyclic ring systems as products in Dieis Ч Alder reactions 411 986
Big-bang theory 6
Bile acids and bile salts 1097Ч1098 1103
Bimolecular elementary step 154 158 164
Bimolecular elimination 214Ч217 (see also УE2 mechanismФ)
Bimolecular nucleophilic substitution see У mechanismФ mechanismФ
Bioenergetics 1162Ч1164
Biological isoprene unit see УIsopentenyl pyrophosphateФ
Biosynthesis of amino acids, by transamination 1123Ч1125
Biosynthesis of cholesterol 1093Ч1095
Biosynthesis of ethylene 189
Biosynthesis of fatty acids 1075Ч1077
Biosynthesis of organohalogen compounds 767
Biosynthesis of phenols 1001Ч1002
Biosynthesis of prostaglandins 1081
Biosynthesis of terpenes 1087Ч1093
Biot, Jean Baptiste 287
Biphenyl 434 497 517
Birch reduction 438Ч439 440 464Ч465
Birch, Arthur J. 439
| Bisabolene 1104
Bloch, Felix 522
Bloch, Konrad 1093Ч1094
Boat conformation of cyclohexane 116Ч117 134
BOC see Уtert-ButoxycarbonyФ
Boiling points and intermolecular attractive forces 80Ч83 147Ч150 708
Boiling points and intramolecular hydrogen bonds 996
Boiling points of alcohols 147Ч149 179 846
Boiling points of alkanes 63 80Ч83 846
Boiling points of alkyl halides 147Ч150 179
Boiling points of amines 918Ч919
Boiling points of carboxylic acids 794
Boiling points of esters 846
Boiling points of thiols 649
Bond angles acetaldehyde 706
Bond angles acetone 706
Bond angles acetylene 365Ч366 367
Bond angles ammonia 31
Bond angles and electron-pair repulsions 29
Bond angles aniline 916
Bond angles benzene 427
Bond angles boron trifluoride 31
Bond angles carbon dioxide 31
Bond angles cyclohexane 116
Bond angles cyclopropane 113 114
Bond angles dialkyl ethers 667
Bond angles enol of 2,4-pentanedione 762
Bond angles ethane 64 367
Bond angles ethylene 38Ч40 90 191 367
Bond angles ethylene oxide 667
Bond angles formaldehyde 31 706
Bond angles formic acid 793
Bond angles methane 31 64Ч65
Bond angles methanol 146Ч147 667 994
Bond angles methylamine 916 917
Bond angles phenol 994
Bond angles water 31 667
Bond angles [10]annulene 454
Bond dissociation energy 12 169Ч172 174
Bond dissociation energy 2-methylpropane 170Ч171 439
Bond dissociation energy acetylene 367
Bond dissociation energy and halogenation of methane 174
Bond dissociation energy aryl halides 972
Bond dissociation energy benzene 972
Bond dissociation energy ethane 169 170 367 972
Bond dissociation energy ethyl halides 972
Bond dissociation energy ethylene 191 367 972
Bond dissociation energy peroxides 242
Bond dissociation energy propane 169 170
Bond dissociation energy propene 395 439
Bond dissociation energy table 170
Bond dissociation energy vinyl halides 972
Bond distances 1,3-butadiene 400
Bond distances acetic acid 797
Bond distances acetylene 92 365Ч366 367
Bond distances alkyl halides 146
Bond distances allene 402
Bond distances ammonium acetate 797
Bond distances and strain 111 132
Bond distances benzene 427
Bond distances carbon-chlorine 834
Bond distances cyclobutadiene 451
Bond distances cyclooctatetraene 450
Bond distances dimethyl ether 667
Bond distances enol of 2,4-pentanedione 762
Bond distances ethane 64 367
Bond distances ethyl chloride 972
Bond distances ethylene 90 191 367
Bond distances ethylene oxide 667
Bond distances formic acid 793
Bond distances methane 63
Bond distances methanol 146
Bond distances methylamine 916 917
Bond distances phenol 994
Bond distances propene 191 367
Bond distances propyne 367
Bond distances vinyl halides 972
Bond lengths see УBond distancesФ
Bond-line formulas 21 68 191
Bonding in  -unsaturated aldehydes and ketones 775-777 -unsaturated aldehydes and ketones 775-777
Bonding in acetylene 14 92Ч93 99 365Ч367 382
Bonding in alcohols 146Ч147
Bonding in aldehydes and ketones 706Ч708 742
Bonding in alkenes 89Ч92 99 190Ч192 220
Bonding in alkyl halides 146Ч147
Bonding in alkynes 365Ч367 382
Bonding in allene 402Ч404
Bonding in amines 916Ч918
Bonding in aryl halides 971Ч972
Bonding in benzene 427Ч428 430Ч431 452Ч453
Bonding in benzyne 982 984
Bonding in carbocations 160Ч162
Bonding in carboxylic acid derivatives 833Ч836
Bonding in carboxylic acids 793Ч794
Bonding in conjugated dienes 400
Bonding in ethane 67 95
Bonding in ethers and epoxides 667
Bonding in ethylene 14 89Ч91 99 190Ч191
Bonding in formaldehyde 14 706
Bonding in free radicals 167Ч168
Bonding in hydrogen 12 58Ч63
Bonding in methane 13 63Ч65
Bonding in phenols 994Ч995
Bonding models, comparison of 63 92Ч93 99
Bonds  in acetylene 92Ч93 99 365Ч366 in acetylene 92Ч93 99 365Ч366
Bonds  in ethylene 89Ч92 99 190Ч191 220 in ethylene 89Ч92 99 190Ч191 220
Bonds  in formaldehyde 706 in formaldehyde 706
Bonds axial and equatorial 117Ч120 135
Bonds bent, in cyclopropane 114
Bonds carbon-metal 587Ч589
Bonds covalent 12Ч13
Bonds double 14 191 220
Bonds hydrogen bonds 148 150Ч151 668
Bonds ionic 10Ч12
Bonds partial 155
Bonds polar  in acetylene 92Ч93 99 365Ч366 in acetylene 92Ч93 99 365Ч366
Bonds polar  in ethylene 89Ч92 99 190Ч191 220 in ethylene 89Ч92 99 190Ч191 220
Bonds polar  in methane and alkanes 63Ч65 67 in methane and alkanes 63Ч65 67
Bonds polar covalent 14Ч17
Bonds polar dipole moments of 17 table
Bonds polar three-center two-electron 252
Bonds polar triple 14 365Ч366
Borane 251
Borneol 1090
Borodin, Aleksandr 769
Borohydride ion 19 (see also УSodium borohydrideФ)
Boron trifluoride Lewis acid/base complex with diethyl ether 46
Boron trifluoride VSEPR and molecular geometry of 30
Bradykinin 1135
Branched-chain carbohydrates 1043
Brevicomin 748
Broadband decoupling 553
Bromination 3-benzyl-2,6-dimethylphenol 1004
Bromination 4-chlor o-N-me thylaniline 503
Bromination aniline 497 950
Bromination anisole 495
Bromination benzene 475 480Ч481 504
Bromination benzylic, of alkylbenzenes 442Ч443 466
Bromination electrophilic aromatic substitution acetophenone 504
Bromination m-fluorophenol 1002
Bromination nitrobenzene 500 973
Bromination of aldehydes 757Ч759
Bromination of alkanes 177Ч178 180
Bromination of alkenes electrophilic 254Ч259 273 307Ч310 447-448
Bromination of alkenes free-radical 396Ч398 416
Bromination of alkynes 381
Bromination of benzene 475 480Ч481
Bromination of carboxylic acids 815Ч816 823
Bromination of conjugated dienes 407
Bromination of ketones 757Ч759 782
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



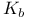 and
and 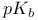
 of
of 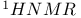 spectrum
spectrum  mechanismФ
mechanismФ -unsaturated aldehydes and ketones
-unsaturated aldehydes and ketones in acetylene
in acetylene in acetylene
in acetylene