|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
Structural formulas Fischer projections 293Ч295 316Ч317 1027Ч1028 1031 1061 1115Ч1116 1150
Structural formulas Lewis dot structures 12
Structural formulas Newman projections 105Ч106 109
Structural formulas of organic molecules 21Ч23
Structural formulas sawhorse 105Ч106
Structural formulas wedge-and-dash 29 105Ч106
Structural isomers see УConstitutional isomersФ
Structural theory 3
Styrene 432
Styrene addition of bromine 447
Styrene addition of hydrogen bromide 448 466
Styrene industrial preparation of 269 424 446 483
Styrene polymers 270 449 1141Ч1142
Styrene polymers copolymer with 1,3-butadiene 408
Substituent effects (see also УField effectФ; УInductive effectФ; УSteric effectsФ)
Substituent effects on acidity of carboxylic acids 801Ч804
Substituent effects on acidity of phenols 998Ч999
Substituent effects on basicity of amines 919Ч923
Substituent effects on equilibrium, hydration of aldehydes and ketones 712Ч717
Substituent effects on rate and regioselectivity in electrophilic aromatic substitution 488Ч508 980
Substituent effects on rate of acid-catalyzed hydration 249
Substituent effects on rate of bimolecular nucleophilic substitution 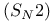 334Ч336 356 334Ч336 356
Substituent effects on rate of bromine addition to alkenes 259
Substituent effects on rate of epoxidation 262
Substituent effects on rate of nucleophilic aromatic substitution 975Ч980
Substituent effects on rate of unimolecular elimination 217Ч218
Substituent effects on rate of unimolecular nucleophilic substitution 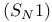 162Ч163 339Ч342 356 391Ч392 445 162Ч163 339Ч342 356 391Ч392 445
Substituent effects on stability of aldehydes and ketones 708
Substituent effects on stability of alkenes 197Ч200 221
Substituent effects on stability of carbocations 160Ч163 181 392 444Ч446
Substituent effects on stability of carbon-carbon triple bonds 374
Substituent effects on stability of free radicals 167Ч172 181 439 441
Substitution reactions 46 153Ч165 326Ч362
Substitution reactions allylic free radical 396Ч398 416
Substitution reactions allylic nucleophilic 393Ч394 416
Substitution reactions benzylic free radical 439 441Ч443 466
Substitution reactions benzylic nucleophilic 444Ч446 465
Substitution reactions electrophilic aromatic 473Ч518
Substitution reactions nucleophilic acyl 830Ч885
Substitution reactions nucleophilic aliphatic 160Ч161 163Ч165 326Ч362
Substitution reactions nucleophilic aromatic 975Ч985 986Ч987 1009
Substitution reactions of aryl diazonium salts 946Ч950 960Ч961
Substitutive nomenclature 144Ч145 178
Succinic acid 202 862
Succinic anhydride 486 862
Succinimide 397 443 862
Sucralose 1051Ч1052
Sucrose 1027 1048 1054
Sucrose octaacetate 1064
Sulfa drugs 951Ч952
Sulfanilamide 951
Sulfenic acids 650
Sulfhydryl group 648
Sulfides alkylation of 686Ч688 695
Sulfides oxidation of 685Ч686 695
Sulfides preparation of 650 685 694Ч695
Sulfinic acids 650
Sulfonate esters nucleophilic substitution reactions of 350Ч353 357
Sulfonate esters preparation of 351 357 636
Sulfonation of 1,2,4,5-tetramethylbenzene 510
Sulfonation of 2,6-dimethylphenol 1003
Sulfonation of benzene 475 478-480
Sulfonation of benzenesulfonic acid 500
Sulfones 686 695
Sulfonic acids 351 475 650
Sulfonium salts 686Ч688 695
Sulfoxides (see also УDimethyl sulfoxide as solventФ)
Sulfoxides optically active 314
Sulfoxides preparation of 685Ч686 695
Sulfur trioxide 479
Sulfuric acid (see also УSulfonationФ)
Sulfuric acid  of 36 of 36
Sulfuric acid as catalyst for alcohol dehydration 203
Sulfuric acid as catalyst for dimerization of alkenes 266Ч267
Sulfuric acid as catalyst for Fischer esterification 638
Sulfuric acid as catalyst for hydration of alkenes 247Ч250 272
Sulfuric acid as catalyst for nitration of arenes 478
Sulfuric acid esters of 641
Sulfuric acid, addition to alkenes 245Ч247 272
Supercoiled DNA 1170Ч1172
syn Periplanar 217
Syndiotactic polymer 313Ч314 318
Synthon 895
Systeme International dТUnitds see УSI unitsФ
Talaromycin A 748
Tariric acid 364
Tartaric acids 310
Tautomerism see УKeto-enol tautomerismФ
Teflon 14 270
Terephthalic acid see У1 acidФ
Termination step 173
Terpenes 1084Ч1093 1102
Terpenes and isoprene rule 1084Ч1086
Terpenes biosynthesis of 1087Ч1093
Terpenes classification 1085
tert-Butoxycarbonyl, protecting group in peptide synthesis 1138Ч1139 1143 1151
tert-Butyl alcohol see also У2-Methyl-2-propanolФ
tert-Butyl alcohol, acidity of 37
tert-Butyl alcohol, dehydration of 203 207
tert-Butyl alcohol, esterification of 656 839
tert-Butyl alcohol, reaction with hydrogen chloride 152 153Ч160
tert-Butyl bromide, nucleophilic substitution in 339Ч341
tert-Butyl cation, electrostatic potential map 142
tert-Butyl cation, intermediate in acid-catalyzed hydration of 2-methylpropene 248
tert-Butyl cation, intermediate in dehydration of tert-butyl alcohol 207
tert-Butyl cation, intermediate in Friedel Ч Crafts alkylation of benzene 481Ч483
tert-Butyl cation, intermediate in nucleophilic substitution 339Ч341
tert-Butyl cation, intermediate in reaction of tert-butyl alcohol with hydrogen chloride 154 156Ч159
tert-Butyl cation, stability of 160
tert-Butyl chloride see also У2-Chloro-2-methylpropaneФ
tert-Butyl chloride by chlorination of 2-methylpropane 177
tert-Butyl chloride in Friedel Ч Crafts reaction 475 481Ч483
tert-Butyl chloride preparation from tert-butyl alcohol 152 153Ч160
tert-Butyl chloride reaction with lithium 589
tert-Butyl chloride solvolysis of 345Ч346 391
tert-Butyl group 74 (see also У1 groupФ)
tert-Butyl group large size of 123 124 128Ч129 200 334Ч335
tert-Butyl hydroperoxide 634Ч635 654
tert-Butyl methyl ether 672
tert-Butyl radical 168 171
tert-Butyl, alcohol,  of 37 40 of 37 40
tert-Butylcyclohexane, conformations 124
tert-Butyllithium 589
tert-Butyloxonium ion intermediate in hydration of 2-methylpropene 248
tert-Butyloxonium ion intermediate in hydration of dehydration of tert-butyl alcohol 207
tert-Butyloxonium ion intermediate in hydration of hydrolysis of tert-butyl bromide 339Ч341
tert-Butyloxonium ion intermediate in hydration of reaction of tert-butyl alcohol with hydrogen chloride 154Ч157 159Ч160
Tertiary carbon 74
Tertiary structure 1145Ч1148
Tesla unit of magnetic field strength 522
Tesla, Nikola 522
Testosterone 1100
Tetrachloromethane 150 166Ч167
Tetrafluoroethylene 14
Tetrafluoromethane 13
Tetrahedral geometry and 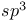 hybridization 64Ч65 hybridization 64Ч65
Tetrahedral geometry and VSEPR 29Ч31 49
Tetrahedral intermediate 811 831 837
Tetrahedral intermediate Claisen condensation 888
Tetrahedral intermediate Dieckmann condensation 890
Tetrahedral intermediate Fischer esterification 812Ч813 823
Tetrahedral intermediate in hydrolysis of acyl chlorides 840Ч841
Tetrahedral intermediate in hydrolysis of amides 864 866
Tetrahedral intermediate in hydrolysis of carboxylic acid anhydrides 844Ч845
Tetrahedral intermediate in hydrolysis of esters 850Ч852 856 876
Tetrahedral intermediate in reaction of esters with ammonia 858
Tetrahydrofuran 132 666
Tetrahydrofuran as solvent 591
Tetrahydrofuran, acid-catalyzed cleavage 676
Tetrahydrofuran, complex with borane 251
| Tetrahydrofuran, dipole moment of 668
Tetrahydropyran 666 667
Tetrahymanol 1104
Tetramethylsilane 525 526 549
Tetramethylsilane, electrostatic potential map 519
Tetrapeptide 1109
Tetraterpene 1085
Thalidomide 296
Theobromine 1158
Thermochemistry 86
Thermodynamic control addition of hydrogen bromide to 1,3-butadiene 407
Thermodynamic control addition to  unsaturated aldehydes and ketones 777Ч778 unsaturated aldehydes and ketones 777Ч778
Thermodynamic control Fries rearrangement 1006
Thermodynamic control glycoside formation 1046
Thermodynamic control Kolbe Ч Schmitt reaction 1006Ч1008
Thiazole 461
Thiirane 666
Thioesters acetyl coenzyme A 1070Ч1071
Thioesters nucleophilic acyl substitution in 835 858Ч859
Thiols acidity of 36 649 655 685
Thiols conjugate addition to  unsaturated carbonyl compounds 778 unsaturated carbonyl compounds 778
Thiols NMR spectra of 652
Thiols oxidation of 650Ч651 657
Thiols physical properties of 648Ч649
Thiols preparation of 648
Thionyl chloride 19
Thionyl chloride, reactions of carboxylic acids 485 810 838
Thionyl chloride, reactions of with alcohols 165 180 636
Thiopental sodium 901
Thiophene 460
Thiophene bonding in 463
Thiophene, electrophilic aromatic substitution in 508
Thiourea 901
Threo, stereochemical prefix 301Ч302
Thromboxanes 1081Ч1082
Thymidine 1159
Thymine 1157 1166
Thymol 1001
Thyroxine 275 297 974
Tin, reduction of nitro groups by 932 957
Toluene 423 424
Toluene benzylic halogenation of 442
Toluene bond dissociation energy 439
Toluene nitration of 488Ч492 506
Toluene oxidation of 444
Toluene physical properties of 995
Torsion angle 105Ч107
Torsional strain 107 133
Torsional strain, boat conformation of cyclohexane 116Ч117
Torsional strain, cyclobutane 115
Torsional strain, cyclopentane 115
Torsional strain, cyclopropane 114
Torsional strain, eclipsed conformation of butane 109Ч110 112
Torsional strain, eclipsed conformation of ethane 107
Tosylates see Уp-Toluenesulfonic acid estersФ
trans-Cycloheptene 201
Transamination 1123Ч1125
Transcription 1172 1174Ч1175 1188
Transfer RNA see УRibonucleic acid transferФ
Transition metal organometallic compounds 608Ч610 616Ч617
Transition state addition of bromine to alkenes 259
Transition state and activation energy 108
Transition state bimolecular elimination (E2) 214Ч215
Transition state bimolecular nucleophilic substitution 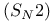 163Ч165 331 333 354 356 163Ч165 331 333 354 356
Transition state bimolecular nucleophilic substitution 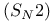 electrostatic potential map 326 electrostatic potential map 326
Transition state bond rotation in ethane 108
Transition state carbocation rearrangement 209Ч210
Transition state conversion of primary alcohols to primary alkyl halides 163Ч165 181 354
Transition state Dieis Ч Alder reaction 409
Transition state double-bond rotation 193
Transition state epoxide ring opening 680 681Ч682
Transition state free-radical halogenation 176
Transition state nucleophilic capture of carbocation 158 159 341
Transition state oxonium ion dissociation 156Ч157 159 162Ч163
Transition state proton transfer 155 159
Transition state unimolecular nucleophilic substitution  156Ч159 341 156Ч159 341
Translation 1172 1177 1178Ч1179
Tranylcypromine 962
Triacylglycerols see УGlycerol estersФ)
Tribromomethane see also УBromoformФ
Tribromomethane, dibromocarbene from 606Ч607
Tricarboxylic acid cycle 1123Ч1124
Trichloroacetic acid 801 802
Trichloromethane 166Ч167 (see also УChloroformФ)
Trichloromethane, boiling point of 150
Triethylamine 920
Trifluoroacetic acid 822
Trifluoroacetic acid, acidity of 41
Triglycerides see УGlycerol estersФ
Trigonal planar geometry and 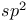 hybridization 89Ч91 157 191 430 706Ч707 hybridization 89Ч91 157 191 430 706Ч707
Trigonal planar geometry and VSEPR 30
Trigonal pyramidal geometry 30
Trimer 266
Trimethyl phosphate 641
Trimethyl phosphite 641
Trimethylamine 918
Trimethylamine H chemical shift 526
Trimyristin 853
Triose phosphate isomerase 1058
Tripeptide 1109
Triphenylamine 921
Triphenylmethane 621
Triphenylmethyl perchlorate 446
Triphenylphosphine 733
Triple bond 14 92Ч94 99 363 365Ч367
Triple bond in benzyne 982 984
Tristearin 846 1071Ч1072
Triterpenes 1085
Triterpenes, biosynthesis of 684Ч685 1089 1093Ч1095
Trityl see УTriphenylmethyl perchlorateФ
Trivial names see УCommon namesФ
Tropylium cation see УCycloheptatrienyl cationФ
Trypsin 1130
Twist-boat see УSkew boat conformation of cyclohexaneФ
Tyrian purple 4 54 974
Ubiquinone 1013
Ultraviolet-visible spectroscopy 565Ч567 577
Ultraviolet-visible spectroscopy alcohols 652
Ultraviolet-visible spectroscopy aldehydes and ketones 741
Ultraviolet-visible spectroscopy amines 953
Ultraviolet-visible spectroscopy carboxylic acids and derivatives 821 874
Ultraviolet-visible spectroscopy ethers and epoxides 691
Ultraviolet-visible spectroscopy phenols 1015
Unimolecular elementary step 156 159Ч160
Unimolecular elimination 206Ч208 217Ч219 223
Unimolecular nucleophilic substitution 160Ч161 180 339Ч345
Uracil 1157
Urea from ammonium cyanate 2
Urea, electrostatic potential map 1
Urea, industrial synthesis of 861
Urea, reaction of, with diethyl malonate 900
Urey, Harold C. 810
Uridine 1159
Uronic acids 1055
valence electrons 9
Valence electrons and Lewis structures 19Ч20
Valence-bond theory 60Ч61 92Ч93 95
Valence-shell electron pair repulsion and molecular geometry 29Ч31 49
van der Waals forces attractive 81Ч83
van der Waals forces attractive and stability of isomeric alkanes 87
van der Waals forces repulsive 82 109Ч110 117 121
van der Waals forces repulsive in stereoisomers 124Ч125 199Ч200 221
van der Waals radius 82 109
van der Waals strain 109 133
van der Waals strain 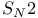 reactions 334Ч336 reactions 334Ч336
van der Waals strain alkenes 199Ч200 221
van der Waals strain axial substituents in cyclohexane 120Ч124 135
van der Waals strain boat conformation of cyclohexane 117
van der Waals strain butane 109 111 133Ч134
van der Waals strain in stereoisomers 124Ч125 199Ч200 221
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



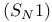
 of
of 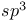 hybridization
hybridization unsaturated aldehydes and ketones
unsaturated aldehydes and ketones 
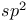 hybridization
hybridization 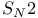 reactions
reactions