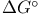|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
Homologous series 68 84
Homolytic bond cleavage 169
Htickel, Erich 451
HtickelТs rule 451Ч460 462Ч463 467
Huffman, Donald 436
Hughes, Edward D. 330 339 361
HundТs rule 9
Hybrid orbitals see УOrbital hybridizationФ
Hydration of aldehydes and ketones, equilibria in 712Ч717 743
Hydration of alkenes acid-catalyzed 247Ч250 272 626
Hydration of alkenes enzyme-catalyzed, of fumaric acid 299Ч300
Hydration of alkenes hydroboration-oxidation 250Ч254 273 626
Hydration of alkenes of alkynes 379Ч381 385 710
Hydrazine cleavage of peptides 1154
Hydrazine in Wolff Ч Kishner reduction 487 713
Hydrazine reaction with aldehydes and ketones 727
Hydrazine reaction with N-alkylphthalimides 930
Hydrazones 727
Hydride shift alcohol dehydration 210Ч211 222
Hydride shift cholesterol biosynthesis 1094Ч1095
Hydride shift electrophilic addition to alkenes 241Ч242
Hydride shift in  reactions 345 reactions 345
Hydride shift in reaction of alcohols with hydrogen halides 355
Hydroboration-oxidation 250Ч254 273 626
Hydroformylation 711Ч712 787
Hydrogen see also УHydrogenationФ; УNuclear magnetic resonance spectroscopyФ
Hydrogen bonding 148
Hydrogen bonding and solvent effects on rate of nucleophilic substitution 346Ч347
Hydrogen bonding between ethers and water 668
Hydrogen bonding in 12 60Ч63
Hydrogen bonding in alcohols 148Ч151 179
Hydrogen bonding in amines 918Ч919
Hydrogen bonding in carboxylic acids 794
Hydrogen bonding in DNA 1168Ч1169
Hydrogen bonding in phenols 995Ч996
Hydrogen bonding intramolecular in enol of 2,4-pentanedione 762
Hydrogen bonding intramolecular in o-nitrophenol 996
Hydrogen bonding intramolecular in peroxyacetic acid 262
Hydrogen bonding intramolecular in salicylate ion 1007
Hydrogen bonding peptides and proteins 1143Ч1145
Hydrogen bromide  of 36 39 43 of 36 39 43
Hydrogen bromide acidity of 43
Hydrogen bromide electrophilic addition to alkenes 235Ч238
Hydrogen bromide electrophilic addition to alkynes 377 385
Hydrogen bromide electrophilic addition to conjugated dienes 405Ч407
Hydrogen bromide electrophilic addition to styrene 466
Hydrogen bromide free-radical addition to alkenes 242Ч245 274 448
Hydrogen bromide free-radical addition to alkynes 379
Hydrogen bromide reaction of with alcohols 151Ч152 164Ч165 180 354Ч355 636
Hydrogen bromide reaction of with epoxides 681 683
Hydrogen bromide reaction of with ethers 674Ч676 692 1010
Hydrogen carbonate ion see УBicarbonateФ
Hydrogen chloride  of 36 39 of 36 39
Hydrogen chloride addition of to alkenes 235 238 241Ч242 271Ч272
Hydrogen chloride electrostatic potential map of 236
Hydrogen chloride reaction with alcohols 151Ч153 153Ч160 180 355
Hydrogen chloride to alkynes 379
Hydrogen chloride to conjugated dienes 405 417
Hydrogen cyanide  of 33 36 of 33 36
Hydrogen cyanide acidity of 33 36 349 777
Hydrogen cyanide addition to  -unsaturated aldehydes and ketones 777 -unsaturated aldehydes and ketones 777
Hydrogen cyanide addition to aldehydes and ketones 717Ч720 743 867
Hydrogen cyanide geometry of 31
Hydrogen cyanide in Kiliani Ч Fischer synthesis 1055Ч1056 1063
Hydrogen cyanide Lewis structure 14
Hydrogen fluoride 13 15
Hydrogen fluoride  of 36 39 of 36 39
Hydrogen fluoride addition to alkynes 378
Hydrogen formation of 6
Hydrogen halides see also УHydrogen bromideФ; УHydrogen chlorideФ; УHydrogen fluorideФ; УHydrogen iodideФ
Hydrogen halides acidity 39
Hydrogen halides addition of to alkenes 235Ч245 271Ч272
Hydrogen halides addition of to alkenylbenzenes 447Ч448 465
Hydrogen halides addition of to alkynes 377Ч379 385
Hydrogen halides addition of to conjugated dienes 405Ч407 417Ч418
Hydrogen halides reactions of with alcohols 151Ч153 163Ч165 180 354Ч355 357 636
Hydrogen halides with epoxides 681 683
Hydrogen halides with ethers 674Ч676 692 1010Ч1011 1018
Hydrogen iodide  of 36 39 of 36 39
Hydrogen iodide cleavage of ethers 674 1018
Hydrogen iodide reaction with alcohols 151
Hydrogen nuclear spin states 522Ч523
Hydrogen peroxide conformations of 104
Hydrogen peroxide oxidation of dialkyl sulfides by 686
Hydrogen peroxide oxidation of organoboranes by 250Ч251 254Ч255
Hydrogen sulfide acidity of 349
Hydrogen sulfide anion of as a nucleophile 327 328 338 349
Hydrogen sulfide anion of basicity of 349
Hydrogen sulfide boiling point 649
Hydrogen-deuterium exchange in alcohols 186 544Ч545
Hydrogen-deuterium exchange in carboxylic acids 819
Hydrogen-deuterium exchange in cyclopentanone 768
Hydrogenation see also УHeat of hydrogenationФ; УHydrogenolysisФ
Hydrogenation catalysts for 230Ч231 374Ч375
Hydrogenation mechanism 232 1074
Hydrogenation of aldehydes and ketones 627Ч628 654
Hydrogenation of alkadienes 399Ч400
Hydrogenation of alkenes 230Ч235 271
Hydrogenation of alkenylbenzenes 447Ч448 466
Hydrogenation of alkyl azides 931
Hydrogenation of alkynes 374Ч375 384
Hydrogenation of benzene 428Ч429
Hydrogenation of carbohydrates 1052 1063
Hydrogenation of carbon monoxide 624
Hydrogenation of esters 632
Hydrogenation of imines 934Ч935
Hydrogenation of ketones 628 654
Hydrogenation of nitriles 932
Hydrogenation of nitroarenes 932
Hydrogenation of vege tableoils 1072 1074
Hydrogenation stereochemistry of 234Ч235 309Ч310
Hydrogenolysis, of benzyl esters 1137Ч1139
Hydrolysis of 2-bromooctane, stereochemistry of 331Ч333 343-344
Hydrolysis of a-bromo carboxylic acids 816
Hydrolysis of acetals 722Ч723 724
Hydrolysis of acyl chlorides 839 840
Hydrolysis of alkyl halides 336Ч337 339Ч342 626
Hydrolysis of alkyl hydrogen sulfates 246
Hydrolysis of amides 862Ч867 941Ч942
Hydrolysis of carboxylic acid anhydrides 843
Hydrolysis of carboxylic acid derivatives, relative rate 833 table
Hydrolysis of cyanohydrins 809
Hydrolysis of epoxides 681Ч682 684
Hydrolysis of esters 849Ч856 876
Hydrolysis of nitriles 808Ч809 822 870Ч871
Hydrolysis of peptides and proteins 1130
Hydrolysis of tert-butyl bromide 339Ч341
Hydronium ion 34 (see also УOxonium ionФ)
Hydronium ion  of 36 43 of 36 43
Hydrophilic 800
Hydrophobic effect 83
Hydroquinone 994 1012
Hydroxide ion as base 45 46 212 369 649 763 797
Hydroxide ion as nucleophile 329 330Ч334 337Ч338 716 766 852Ч856 866
Hydroxylamine 727
Hydroxylation of alkenes anti 683Ч684
Hydroxylation of alkenes syn 635
Hyperconjugation 161Ч162
Hypophosphorous acid 948Ч949 961
Hz (symbol for Hertz), unit of frequency 520
Ibuprofen 183 296 824 1083
Icosane 71
Icosanoic acid 1073
Icosanoids 1080Ч1084
Ignarro, L. J. 1149
Iijima, Sumio 437
Imidazole 461 922Ч923
Imides 862
Imines as intermediates in reductive amination 934Ч935
Imines in addition of Grignard reagents to nitriles 871
| Imines in biological chemistry 728Ч729 1125
Imines preparation of 724Ч727 744
Imines stereoisomers 748
Iminium ion 935
Imino acid 870Ч871
Indene 447Ч448
Index of hydrogen deficiency 574
Indigo 4 113 914
Indole 460Ч461
Induced dipole-induced dipole forces 81Ч83 147Ч150
Inductive effect 41 50 161
Inductive effect and acidity of alcohols 40Ч41
Inductive effect and acidity of carboxylic acids 41 795Ч796 801Ч804
Inductive effect of alkyl groups in aldehydes and ketones 708 713Ч715
Inductive effect of alkyl groups in alkenes 196Ч199 221
Inductive effect of alkyl groups in alkynes 374
Inductive effect of alkyl groups in carbocations 161 181 341Ч342
Inductive effect of alkyl groups of trifluoromethyl group 41 492 715
Industrial preparation 1,2-epoxypropane 678
Industrial preparation 1,3-butadiene 404
Industrial preparation acetaldehyde 644 711
Industrial preparation acetic acid 806
Industrial preparation acetic anhydride 841
Industrial preparation acetone 711 1000 1023
Industrial preparation acetylene 364
Industrial preparation aldehydes 711Ч712
Industrial preparation benzene 424
Industrial preparation chloromethanes 167
Industrial preparation ethylene 189 202
Industrial preparation ethylene oxide 269 644
Industrial preparation formaldehyde 624 711
Industrial preparation isopropyl alcohol 246
Industrial preparation methanol 623Ч624
Industrial preparation phenol 975 1000 1023
Industrial preparation propene 188 202
Industrial preparation styrene 446 483
Industrial preparation terephthalic acid 806
Industrial preparation urea 861
Infrared spectra see also УInfrared spectroscopyФ
Infrared spectra 1-hexene 560Ч561
Infrared spectra 2-hexanol 560 563
Infrared spectra 2-hexanone 563Ч564
Infrared spectra 4-phenylbutanoic acid 820
Infrared spectra butanal 739
Infrared spectra butylamine 952
Infrared spectra cyclohexanol 651 652
Infrared spectra diethylamine 952
Infrared spectra dipropyl ether 688
Infrared spectra hexane 560
Infrared spectra p-cresol 1014
Infrared spectra tert-butylbenzene 560Ч561
Infrared spectroscopy 559Ч564 577
Infrared spectroscopy absorption frequencies table 561
Infrared spectroscopy alcohols 560 561 651
Infrared spectroscopy aldehydes and ketones 561 563 738 739
Infrared spectroscopy amines 951Ч952
Infrared spectroscopy carboxylic acids and derivatives 561 819Ч820 872
Infrared spectroscopy ethers and epoxides 688 690
Infrared spectroscopy nitriles 561 872
Infrared spectroscopy phenols 1014
Ingold, Sir Christopher 3
Ingold, Sir Christopher and stereochemical notation 194 290Ч293
Ingold, Sir Christopher and studies of reaction mechanisms electrophilic aromatic substitution 477
Ingold, Sir Christopher and studies of reaction mechanisms elimination 214Ч216
Ingold, Sir Christopher and studies of reaction mechanisms nucleophilic substitution 160 164 330 339
Initiation step 167 172 243Ч244 268
Initiators of free-radical reactions 242Ч244 268 442-443
Insulin 1129 1131Ч1133 1141
Integration and NMR peak area measurement 532Ч533
International Union of Pure and Applied Chemistry see ФIUPACФ
Inversion of configuration complete, in  reactions 331Ч333 356 reactions 331Ч333 356
Inversion of configuration partial,  reactions 342Ч344 356 reactions 342Ч344 356
Iodination 166 256 480
iodobenzene 604 973
Iodomethane see УMethyl iodideФ
Iodomethylzinc iodide 604Ч606 615 616
Ion-exchange chromatography 1130
Ionic bonds 10Ч12 47
Ionization constant see УAcid dissociation constantsФ
Ionization energy 11
Ionization potential see УIonization energyФ
Ionophore 670 1078Ч1079
Iron(III) salts as catalysts in halogenation of arenes 475 480Ч481
Iron, reduction of nitroarenes by 932
Isoamyl acetate, in bananas 183 845
Isobutane 67 (see also У2-MethylpropaneФ)
Isobutene see У2-MethylpropeneФ
Isobutyl chloride 177 482
Isobutyl group 74 (see also У2-Methylpropyl groupФ)
Isobutylene 187 (see also У2-MethylpropeneФ
Isocitric acid 828
Isoelectric point 1118Ч1120
Isoelectronic 50
Isoionic point see УIsoelectric pointФ
Isolated diene 398 416
Isomerism see УIsomersФ
Isomers 2 48
Isomers, alkanes 67 69Ч70 71Ч73 96
Isomers, alkenes 192Ч194 220
Isomers, classification 315 table
Isomers, constitutional 23 48 67
Isomers, keto-enol 379 759Ч761
Isomers, number of 69
Isomers, stereoisomers see УStereoisomersФ
Isopentane 69Ч70 (see also У2-MethylbutaneФ
Isopentenyl pyrophophate 1087Ч1093 1102
Isoprene 408 1084
Isoprene rule 1084Ч1086
Isoprenoid compounds see УTerpenesФ
Isopropenyl group 190
Isopropyl alcohol 20 145
Isopropyl alcohol  of 37 40 of 37 40
Isopropyl alcohol industrial preparation of 246
Isopropyl alcohol properties of 624
Isopropyl chloride, 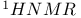 spectrum 540 spectrum 540
Isopropyl group 74 (see also У1-Methylethyl groupФ)
Isopropyl group size of 123 334Ч335
Isopropyl group spin-spin splitting in 540
Isopropyl hydrogen sulfate 245Ч246
Isopropyl radical 169 170
Isopropylbenzene see also УCumeneФ
Isopropylbenzene conversion to phenol 1000 1023
Isopropylbenzene nitration 932
Isopropylbenzene preparation of 931
Isopropylcyclohexane 123
Isoquinoline 460
Isotactic polymers 313Ч314 612
Isotopes see also УCarbonФ; УHydrogen-deuterium exchangeФ
Isotopes H-D exchange in alcohols 186 544Ч545
Isotopes H-D exchange in carboxylic acids 819
Isotopes H-D exchange in cyclopentanone 768
Isotopes in biosynthetic studies 1092Ч1093
Isotopes in study of reaction mechanisms bromine addition to alkenes 256
Isotopes in study of reaction mechanisms Claisen rearrangement 1011
Isotopes in study of reaction mechanisms ester hydrolysis 852 854Ч855
Isotopes in study of reaction mechanisms esterification 810
Isotopes in study of reaction mechanisms hydrolysis of chlorobenzene 985
Isotopes in study of reaction mechanisms nucleophilic aliphatic substitution 361
Isotopes in study of reaction mechanisms nucleophilic aromatic substitution 982 985
Isotopic clusters in mass spectrometry 569Ч570
IUPAC (International Union of Pure and Applied Chemistry) 78 (see also УNomenclatureФ УIUPACФ)
J (symbol for coupling constant) 538
Japanese National Institute of Advanced Industrial Science and Technology 555
Joule (SI unit of energy) 11
K (symbol for equilibrium constant) relation to 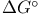 122Ч123 122Ч123
Karplus, Martin 580
Kazan, University of 3
Keku16, August 3 424Ч427
Kendrew, John C. 1146
Ketals see УAcetalsФ
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 reactions
reactions  of
of  -unsaturated aldehydes and ketones
-unsaturated aldehydes and ketones  reactions
reactions 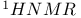 spectrum
spectrum