|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
Regioselectivity hydroboration-oxidation 250Ч254 273
Regioselectivity nucleophilic aromatic substitution 981Ч985
Relative configuration 289
Resolution 310Ч312 317
Resolution enzymatic 312
Resonance 3 24Ч27 49
Resonance  -unsaturated carbonyl compounds 776 -unsaturated carbonyl compounds 776
Resonance  -keto ester anions 887 -keto ester anions 887
Resonance aldehydes and ketones 498 707Ч708
Resonance allyl radical 395
Resonance allylic carbocations 391Ч394
Resonance amides 836 940
Resonance aniline 917
Resonance benzene 427Ч428
Resonance benzylic carbocations 445
Resonance benzylic radicals 441
Resonance carboxylic acid derivatives 833Ч838
Resonance carboxylic acids 794
Resonance cyclohexadienyl anions 977Ч980
Resonance cyclohexadienyl cations 474 489Ч494 496 497 499 501 506Ч507
Resonance energy 1,3,5-hexatriene 429
Resonance energy anthracene 434Ч435
Resonance energy benzene 428Ч429 463
Resonance energy conjugated dienes 399Ч400
Resonance energy cyclooctatetraene 450
Resonance energy naphthalene 434
Resonance energy phenanthrene 434
Resonance energy [18]annulene 455
Resonance enolate ions 763Ч765
Resonance formic acid 794
Resonance ozone 24Ч25 262
Resonance p-nitroaniline 921
Resonance phenol 995
Resonance phenoxide anions 997 999 1007
Resonance protonated benzoic acid 812
Resonance protonated ketone 717
Resonance rules for 26Ч27 table
Resorcinol 994
Resorcinol acetylation 1005
Restriction enzymes 1101
Retention of configuration 254 331Ч332
Retention of configuration in acylation of alcohols 640
Retention of configuration in Baeyer Ч Villiger oxidation 736Ч738
Retention of configuration in ester hydrolysis 855
Retinal 729
Retinol 625 729
Retro-aldol cleavage 1058
Retrosynthetic analysis acetoacetic ester synthesis 895
Retrosynthetic analysis Grignard synthesis of alcohols 598Ч601 616
Retrosynthetic analysis malonic ester synthesis 898
Retrosynthetic analysis Simmons Ч Smith reaction 605
Retrosynthetic analysis Wittig reaction 732Ч733
Reverse transcriptase 1179
Rhodium, hydrogenation catalyst 230 231
Rhodopsin 729
Ribavarin 1160
Ribonuclease 1142
Ribonucleic acid (RNA) 1164 1172Ч1177
Ribonucleic acid (RNA) messenger (mRNA) 1172 1174Ч1175 1178 1188
Ribonucleic acid (RNA) polymerase 1175
Ribonucleic acid (RNA) purine and pyrimidine bases in 1157 1158Ч1160
Ribonucleic acid (RNA) ribosomal (rRNA) 1177
Ribonucleic acid (RNA) transfer (tRNA) 1175Ч1176 1178 1189
Ribosome and rRNA 1177
Ribozyme 1177
Rickets 1097
Ring flipping see УRing inversionФ
Ring inversion cyclohexane 119Ч120 135 544Ч545
Ring inversion substituted cyclohexanes 120Ч124 125Ч129 135Ч136
RNA World 1177
RNA, mRNA, rRNA, and tRNA see УRibonucleic acidФ
Roberts, John D. 982
Robinson annulation 779 783
Robinson, Sir Robert 3 427
Rotamer 105 (see also УConformationФ)
Rotational energy barrier alkenes 193
Rotational energy barrier amides 835
Rotational energy barrier butane 109Ч110
Rotational energy barrier conjugated dienes 401Ч402
Rotational energy barrier ethane 107Ч108
Rubber 408
Rubbing alcohol 21 145
Ruzicka, Leopold 1085
S (symbol for entropy) 122
S-Adenosylmethionine 339 687
s-Cis conformation 401Ч402
s-Trans conformation 401Ч402
Sabatier, Paul 231 591
Sabinene 1107
Saccharic acids see УAldaric acidsФ
Saccharin 1051
Salicylic acid 792
Salicylic acid, acetylation of 1006
Salicylic acid, acidity of 33 1007
Salicylic acid, synthesis of 1006Ч1008
Samuelsson, Bengt 1084
Sandmeyer reactions 946 948 961 973
Sanger, Frederick 1129Ч1133 1180Ч1182
SangerТs reagent see У1-Fluoro-2
Saponification 852Ч856
Sawhorse diagrams 105Ч106
Saytzeff see УZaitsevФ
Schiemann reaction 946 947 960
SchiffТs base 724 744
Schroedinger equation see УWave equationФ
Schroedinger, Erwin 7 1167
Scientific method 239
sec-Butyl alcohol 638 (see also У2-ButanolФ)
sec-Butyl chloride. see У2-ChlorobutaneФ
sec-Butyl group 74 (see also У1-Methylpropyl groupФ)
sec-Butyl methyl ether 674
sec-Butyl phenyl ketone, enolization of 768Ч769
sec-Butyl radical 175Ч176
Secobarbital 900
Seconal 900
Secondary carbon 74
Secondary structure 1143Ч1145
Selectivity see УRegioselectivityФ; УStereoselective reactionsФ
Semicarbazide 727
Semicarbazones 727
Sequence rule and R-S notation 290Ч293 316
Sequence rule application to alkene stereochemistry 193Ч195 220
Serotonin 924Ч925
Sesquiterpene 1085
Sesterpene 1085
Sex attractant see УPheromone sex
Sex hormones 1098Ч1100 1103
Shared-electron pair bond see УCovalent bondФ
Shielding of nuclei in NMR spectroscopy 525Ч532 549Ч551
SI units 11 24
Sickle-cell anemia 1150
Sigma bond 60
Sigmatropic rearrangement 1012
Silk 1144
Silver oxide 938 1012 1018
Simmons Ч Smith reaction (reagent) 605
Simmons, Howard E. 605
Simvastatin 1096
Sinigrin 1044
Sites of unsaturation see УIndex of hydrogen deficiencyФ
Skew-boat conformation of cyclohexane 117 134
Smalley, Richard 436
Smith, Bradley D. 555
Smith, Ronald D. 605
Soap manufacture 853
Soap mode of action 800Ч801
Sodium 1-dodecyl sulfate (SDS) 800 1121
Sodium acetylide 360 588
Sodium acetylide, preparation of 370 371
Sodium acetylide, reaction with alkyl halides 360 371Ч372
| Sodium acetylide, reaction with cyclohexanone 597
Sodium alkoxides as bases in elimination reactions 211Ч212 348Ч350
Sodium alkoxides in Williamson ether synthesis 672Ч673 693
Sodium alkoxides, preparation of 212
Sodium amide as base 370Ч373 383 597
Sodium amide, reaction with aryl halides 981Ч985
Sodium borohydride, reduction of aldehydes and ketones 627Ч631 654 713
Sodium borohydride, reduction of aryl diazonium ions 949
Sodium borohydride, reduction of carbohydrates 1052Ч1053 1063
Sodium cyanoborohydride 935
Sodium dichromate (see also УChromic acid oxidationФ; УPotassium dichromateФ)
Sodium dichromate, oxidation of alcohols 642 657
Sodium dichromate, oxidation of alkylbenzenes 443Ч444 466 506
Sodium ethoxide as base in acetoacetic ester synthesis 894Ч896
Sodium ethoxide as base in Claisen and Dieckmann condensations 887 891
Sodium ethoxide as base in elimination reactions 212 348Ч350
Sodium ethoxide as base in malonic ester synthesis 897Ч899
Sodium ethoxide, reaction with epoxides 679
Sodium hydride 892
Sodium hypochorite 644Ч645
Sodium iodide 329
Sodium lauryl sulfate 800 (see also УSodium 1-dodecyl sulfateФ)
Sodium metaperiodate 685Ч686
Sodium methoxide, reaction with aryl halides 975Ч980
Sodium stearate 799
Sodium, reaction with alkynes 376Ч377 384
Sodium, reaction with arenes 438Ч439 440 464Ч465
Solid-phase peptide synthesis 1141Ч1143
Solvation and nucleophilicity 338
Solvent effects, and rate of nucleophilic substitution 345Ч348 356
Solvolysis of alkyl halides 336Ч339 345
Solvolysis of allylic halides 391Ч394 416
Solvolysis of benzylic halides 444Ч446
Somatostatin 1154
Sondheimer, Franz 456
Sorbitol 658
Space-filling models 28 (see also УMolecular models and modelingФ)
Space-filling models and steric hindrance 335
Specific rotation 288
Spectral Data Base System 555
Spectrometer 521
Spectrometer mass 567Ч568
Spectrometer nuclear magnetic resonance 523Ч524
Spectroscopy 519Ч586 (see also УMass spectrometryФ)
Spectroscopy 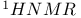 522Ч545 575Ч576 522Ч545 575Ч576
Spectroscopy general principles 520Ч521 575
Spectroscopy infrared 559Ч564 577
Spectroscopy ultraviolet-visible 565Ч567 577
Spectroscopy Web sites 555
speed of light 520
Spermaceti 1079
Spermidine 925
Spermine 925
Spin density in allyl radical 395
Spin density in benzyl radical 441
Spin-spin coupling 537
Spin-spin splitting in 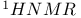 535Ч544 576 535Ч544 576
Spin-spin splitting in  580 580
Spin-spin splitting n + 1 rule 535 544
Spirocyclic hydrocarbons 129Ч130 136
Spiropentane 130
Splitting diagrams AX to AM to AB 541
Splitting diagrams doublet of doublets 543
Splitting diagrams quartet 537
Splitting diagrams triplet 539
Squalene 684 1085 1086 1094 1103
Squalene 2,3-epoxide 684
Squalene 2,3-epoxide in cholesterol biosynthesis 1094 1095
Staggered conformation 105Ч107 133
Stamps 1Ч5
Stanozolol 1099
Starch 1049Ч1050
Stearic acid 792 1073
Sterculic acid 200
Stereocenter see УChirality centerФ
Stereochemistry 281Ч325
Stereochemistry and chemical reactions (see also УStereoselective reactionsФ; УStereospecific reactionsФ)
Stereochemistry and chemical reactions bimolecular nucleophilic substitution 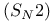 331Ч334 353 356 331Ч334 353 356
Stereochemistry and chemical reactions ester hydrolysis 312 855
Stereochemistry and chemical reactions hydrogenation of alkenes 234Ч235 309
Stereochemistry and chemical reactions that produce chiral molecules 297Ч300 316Ч317
Stereochemistry and chemical reactions that produce diastereomers 307Ч310 317
Stereochemistry and chemical reactions unimolecular nucleophilic substitution  342Ч344 356 342Ч344 356
Stereochemistry Fischer projection formulas  -amino acids 1115Ч1116 1150 -amino acids 1115Ч1116 1150
Stereochemistry Fischer projection formulas carbohydrates 1027Ч1028 1031 1061
Stereochemistry Fischer projection formulas chiral molecules 293Ч295 316
Stereochemistry Fischer projection formulas two chirality centers 300Ч304 317
Stereochemistry notational systems (see also УStereoisomersФ)
Stereochemistry notational systems cis and trans 124 192Ч193 220
Stereochemistry notational systems D and L 293 1027Ч1032 1061 1115Ч1116
Stereochemistry notational systems E and Z 193Ч195 220
Stereochemistry notational systems erythro and threo 301Ч302
Stereochemistry notational systems R and S 290Ч293 316
Stereoelectronic effects bimolecular elimination 216Ч217 223
Stereoelectronic effects nucleophilic substitution 333
Stereogenic axis see УChirality axisФ
Stereogenic center see УChirality centerФ
Stereoisomers 23 124Ч129 135Ч136
Stereoisomers alkenes 192Ч195 220
Stereoisomers diastereomers 300Ч312 317
Stereoisomers enantiomers 281Ч300 316
Stereoisomers endo and exo 734
Stereoisomers epimers 1056
Stereoisomers maximum number of 306Ч307 317
Stereoregular polymers 312Ч314 317Ч318 612
Stereoselective reactions 234 309Ч310
Stereoselective reactions addition to carbonyl groups 734Ч735
Stereoselective reactions alcohol dehydration 206
Stereoselective reactions dehydrohalogenation of alkyl halides 213
Stereoselective reactions enzyme-catalyzed hydration of fumaric acid 299Ч300
Stereoselective reactions hydrogenation of alkenes 234 309Ч310
Stereoselective reactions metal-ammonia reduction of alkynes 376Ч377 384
Stereospecific reactions 308Ч310
Stereospecific reactions Baeyer Ч Villiger oxidation 736Ч738
Stereospecific reactions bimolecular (E2) elimination 216Ч217
Stereospecific reactions bimolecular nucleophilic substitution 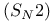 331Ч333 353 356 331Ч333 353 356
Stereospecific reactions Dieis Ч Alder reaction 410
Stereospecific reactions epoxidation of alkenes 260Ч262 273 309 676
Stereospecific reactions epoxide formation from bromohydrins 677
Stereospecific reactions epoxide ring opening 680 683Ч684
Stereospecific reactions halogen addition to alkenes 256Ч259 273 307Ч310
Stereospecific reactions halogen addition to alkynes 381
Stereospecific reactions Hofmann elimination 938
Stereospecific reactions hydroboration of alkenes 252Ч254 273
Stereospecific reactions hydrogenation of alkenes 234 309Ч310
Stereospecific reactions hydrogenation of alkynes 374Ч375 384
Stereospecific reactions hydroxylation of alkenes 635 683Ч684
Stereospecific reactions Simmons Ч Smith reaction 605Ч606
Steric effects 109
Steric effects and stability of isomeric alkenes 197Ч201 221 233
Steric effects and stereoselectivity 309Ч310 734
Steric effects in bimolecular nucleophilic substitution 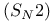 334Ч336 356 334Ч336 356
Steric effects in cyclohexane derivatives 121
Steric effects in electrophilic aromatic substitution 503
Steric effects in Hofmann elimination 940
Steric effects in hydration of aldehydes and ketones 712Ч717
Steric effects in hydroboration of alkenes 252
Steric effects in hydrogenation of a-pinene 234Ч235
Steric effects in sodium borohydride reduction 734
Steric hindrance 109 235 734
Steric hindrance in bimolecular nucleophilic substitution 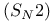 334Ч336 356 334Ч336 356
Steric strain 107 109 200Ч201
Steroids 140 141 306Ч307 1093Ч1100
strain see УAngle strainФ; УTorsional strainФ; Уvan der Waals strainФ
Strain energy minimization 111
Strecker synthesis 1121
Strecker, Adolf 1121
Streptimidone 322
Stretching vibrations and infrared spectroscopy 559
Strong acids and bases definitions 33 44
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 -unsaturated carbonyl compounds
-unsaturated carbonyl compounds  -keto ester anions
-keto ester anions 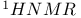

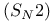

 -amino acids
-amino acids