|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Carey F.A. Ч Organic Chemistry |
|
|
 |
| ѕредметный указатель |
Keto-enol isomerism 379 759Ч761
Keto-enol tautomerism see УKeto-enol isomerismФ
Ketones acidity of 764
Ketones chemical shifts, 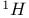 and and 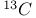 738Ч740 738Ч740
Ketones classification of carbons in 756
Ketones enolization of 757Ч765 782
Ketones infrared absorption frequencies 561 563 738
Ketones naturally occurring 709
Ketones nomenclature of 705 741
Ketones physical properties of 708
Ketones preparation of 709Ч712
Ketones preparation of by acetoacetic ester synthesis 894Ч896 907
Ketones preparation of by decarboxylation of  -keto acids 893 905 -keto acids 893 905
Ketones preparation of by hydration of alkynes 379Ч381 385 710
Ketones preparation of by oxidation of secondary alcohols 642 657 710 711
Ketones preparation of by ozonolysis of alkenes 710
Ketones preparation of from nitriles 871Ч872 877
Ketones reactions of acetal formation 720Ч723 744
Ketones reactions of acylation via enolate 892Ч893 906
Ketones reactions of aldol condensation 773 775 783
Ketones reactions of Baeyer Ч Villiger oxidation 736Ч738 745 847
Ketones reactions of Clemmensen reduction 486Ч488 505 713
Ketones reactions of cyanohydrin formation 717Ч720 743
Ketones reactions of enamine formation 727Ч728 744
Ketones reactions of halogenation 757Ч759
Ketones reactions of hydration 712Ч717 743
Ketones reactions of imine formation 724Ч727 744
Ketones reactions of reduction 627Ч631 654 713
Ketones reactions of reductive amination 934Ч935 957
Ketones reactions of with derivatives of ammonia 727
Ketones reactions of with ester enolates 904
Ketones reactions of with Grignard reagents 596 600 616 713
Ketones reactions of with organolithium reagents 597 616 627 713
Ketones reactions of Wittig reaction 730Ч734 744
Ketones reactions of Wolff Ч Kishner reduction 487 713
Ketones spectroscopy 738Ч741
Ketones structure and bonding 706Ч708 742
Ketoses 1027 1041 1061
Kevlar 868
Kharasch, Morris S. 242Ч243
Kiliani Ч Fischer synthesis 1055Ч1056 1063
Kinetic control 406Ч407
Kinetic control addition to  -unsaturated aldehydes and ketones 778 -unsaturated aldehydes and ketones 778
Kinetic control addition to conjugated dienes 406Ч407
Kinetic control O-acylation of phenols 1006
Kinetic studies of  -halogenation of aldehydes and ketones 758 -halogenation of aldehydes and ketones 758
Kinetic studies of elimination reactions of alkyl halides 214
Kinetic studies of ester hydrolysis 854
Kinetic studies of nucleophilic aromatic substitution 977
Kinetic studies of nucleophilic substitution 330 339Ч342 356
Kolbe Ч Schmitt reaction 1006Ч1008 1017
Kolbe, Hermann 1006
Kossel, Walther 12
Kratschmer, Wolfgang 436
Krebs cycle 1123Ч1124
Kroto, Harold W. 436
L-3,4-Dihydroxyphenylalanine 1126
L-Arabinose 1030 1055Ч1056
L-Arginine 1112 1114 1119
L-Arginine electrostatic potential map 1114
L-Asparagine 1111 1113 1119
L-Asparagine electrostatic potential map 1114
L-Aspartic acid 1112 1115
L-Aspartic acid electrophoresis of 1120Ч1121
L-Aspartic acid electrostatic potential map 1114
L-Aspartic acid isoelectric point 1118 1119
L-Cysteine 1112 1113 1119
L-Cysteine disulfide formation in 1129 1131Ч1133 1146
L-Cysteine electrostatic potential map 1114
L-Daunosamine 1043
L-DOPA see УL-3 4-DihydroxylphenylalanineФ
L-Erythrose 1029
L-Glucose 1056
L-Glutamic acid 1112 1114 1119 1123Ч1125
L-Glutamic acid conversion to glutamine 1163
L-Glutamic acid electrostatic potential map 1114
L-Glutamine 1112 1113 1119
L-Glutamine electrostatic potential map 1114
L-Glutamine formation of 1163
L-Glyceraldehyde 1028
L-Histidine 1112 1114 1119
L-Histidine decarboxylation of 1126
L-Histidine electrostatic potential map 1114
L-Isoleucine 1111 1113 1119
L-Isoleucine electrostatic potential map 1114
L-Leucine 1111 1113 1119
L-Leucine electrostatic potential map 1114
L-Lysine 1112 1114
L-Lysine, electrophoresis of 1120Ч1121
L-Lysine, electrostatic potential map 1114
L-Lysine, isoelectric point 1118 1119
L-Mannose 1056
L-Methionine 687 1111 1119
L-Methionine and protein biosynthesis 1178
L-Methionine electrostatic potential map 1114
L-Phenylalanine 1111 1113 1119
L-Phenylalanine in PKU disease 1124Ч1125
L-Phenylalanine, electrostatic potential map 1114
L-Phenylalanine, N-benzyloxycarbonyl derivative 1137Ч1138
L-proline 1110 1111 1113 1119 1144
L-Proline electrostatic potential map 1114
L-Rhamnonolactone 1063
L-Rhamnose 1063
L-Serine 1112 1113 1119
L-Serine electrostatic potential map 1114
L-Threonine 1112 1113 1119
L-Threonine, electrostatic potential map 1114
L-Threose 1029
L-Tryptophan 1111 1113 1119
L-Tryptophan, electrostatic potential map 1114
L-Tyrosine 1111 1113 1119 1124
L-Tyrosine, electrostatic potential map 1114
L-Valine 1111 1113 1119
L-Valine electrostatic potential map 1114
L-Vancosamine 1043
L-Xylulose 1041
Lactams 861Ч862
Lactase 1048
Lactic acid 792 1069
Lactic acid (S) enantiomer by enzymic reduction of pyruvic acid 735
Lactic acid biological oxidation of 647
Lactones 814Ч815 845
Lactones formation of by oxidation of carbohydrates 1054
Lactones formation of in Baeyer Ч Villiger oxidation of cyclic ketones 749
Lactose 1048
Lanosterol 1094Ч1095
Lapworth, Arthur 757
Lauric acid 1073
Lavoisier, Antoine Ч Laurent 1Ч2
LDA see УLithium diisopropylamideФ
Le Bel, Joseph Achille 281
Le CratelierТs principle 249
Leaving groups and their basicity 330 352
Leaving groups halides 214 326Ч327 330 356
Leaving groups in nucleophilic aromatic substitution 977
Leaving groups nitrogen of diazonium ions 944
Leaving groups p-toluenesulfonates 350Ч353
Lecithin see УPhosphatidylcholineФ
Lenthionine 132
Leucine enkephalin 1128Ч1129
Leukotrienes 1081Ч1082
Levorotatory 288
Levulinic acid 828
Lewis acid 45Ч47 143
Lewis acid as electrophile 47 156
Lewis base 45Ч47 143
Lewis base as nucleophile 157Ч158 164 336Ч339
Lewis structural formulas 12Ч21 47
Lewis structural formulas and resonance 24Ч27 49
Lewis structural formulas formal charges in 17Ч21 48
| Lewis structural formulas multiple bonding in 14 48
Lewis structural formulas writing of 20 table
Lewis, Gilbert N. 3 12
Lexan 868
Liege rules 78
Limonene 80 285 1089
Linalool 284
Linamarin 1044 1066
Lindlar palladium 374Ч375 384
Linear  -olefins 610 622 711Ч712 -olefins 610 622 711Ч712
Linoleic acid 1073 1080
Linolenic acid 1073
Lipids 1069Ч1108 (see also УFatsФ; УOilsФ; УPhospholipidsФ; УSteroidsФ; УTerpenesФ; УWaxesФ)
Lipoic acid 132 650
Lipophilic 800
Lipoxygenase 1082
Lister, Joseph 996
Lithium aluminum hydride, reducing agent for aldehydes and ketones 628Ч631 654 713
Lithium aluminum hydride, reducing agent for alkyl azides 931 957
Lithium aluminum hydride, reducing agent for amides 933 957
Lithium aluminum hydride, reducing agent for carboxylic acids 632 654 711 810
Lithium aluminum hydride, reducing agent for epoxides 681
Lithium aluminum hydride, reducing agent for esters 632 654 848
Lithium aluminum hydride, reducing agent for nitriles 932 957
Lithium aluminum hydride, reducing agent for table 654
Lithium dialkylcuprates see УLithium diorganocupratesФ
Lithium diisopropylamide (LDA) 903Ч905
Lithium dimethylcuprate see УLithium diorganocupratesФ
Lithium diorganocuprates conjugate addition to  -unsaturated ketones 780 784 -unsaturated ketones 780 784
Lithium diorganocuprates preparation of 602Ч603 615
Lithium diorganocuprates reactions with alkenyl, alkyl, and aryl halides 603Ч604 617
Lithium electronegativity 15 588
Lithium hydride, electrostatic potential map of 16
Lithium reaction with alkyl and aryl halides 589Ч590 615
Lithium reduction of alkynes 376Ч377
Locant, numerical prefix in IUPAC nomenclature of 72 188
London dispersion forces see Уvan der Waals forcesФ
Lovastatin 1096
Low-density lipoprotein 1096
Lowry, Thomas M. 33
Luciferin 461
Lucite 883
Lycopene 566Ч567 1100
Lynen, Feodor 1093Ч1094
m-Cresol 993
m-Cresol 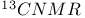 spectrum 551Ч552 1014Ч1015 spectrum 551Ч552 1014Ч1015
m-Cresol acidity of 998
m-Fluorophenol, bromination 1002
m-Nitroaniline, diazotization of 948 959 960
m-Nitrophenol acidity of 998 999
m-Nitrophenol preparation of 960 1001
m-Xylene 432Ч433
m-Xylene nitration of 503
Macrolide antibiotics 814
Magnesium, reaction of with alkyl and aryl halides 591Ч592 615
Magnetic field induced, and nuclear shielding 526Ч532
Magnetic field strength of 522Ч523
Magnetic resonance imaging (MRI) 546
Maleic anhydride 841 842
Maleic anhydride dienophile in Dieis Ч Alder reaction 410 418
Malonic acid 792
Malonic acid acidity of 804
Malonic acid decarboxylation of 816Ч818 824
Malonic ester synthesis 897Ч900 907
Malonyl coenzyme A 1075Ч1076 1091
Maltase 1047
Maltose 1046Ч1047 1053
Mandelic acid 792
Markovnikov, Vladimir 237
MarkovnikovТs rule 237
MarkovnikovТs rule in addition to alkenes 236Ч241
MarkovnikovТs rule in addition to alkynes 377Ч379 380 385
Mass spectrometer 567Ч568
Mass spectrometry 567Ч573 577
Mass spectrometry alcohols 652Ч653
Mass spectrometry aldehydes and ketones 741
Mass spectrometry amines 953
Mass spectrometry and gas chromatography 572Ч573
Mass spectrometry carboxylic acid derivatives 873Ч874
Mass spectrometry ethers 691
Mass spectrometry phenols 1015Ч1016
Mass-to-charge ratio (m/z) 568
Mauveine 4
Maxam, Allan 1181
Mayo, Frank R. 243
McGwire, Mark 1099
Mechanism 3
Mechanism 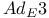 378 378
Mechanism acetal formation 720Ч721 1044
Mechanism Aldol addition 770
Mechanism Baeyer Ч Villiger oxidation 737
Mechanism biosynthesis of amino acids by transamination 1125
Mechanism biosynthesis of cholesterol 1093Ч1095
Mechanism biosynthesis of fatty acids 1075Ч1077
Mechanism biosynthesis terpenes 1087Ч1093
Mechanism Birch reduction 440
Mechanism borohydride reduction of aldehydes and ketones 630
Mechanism chlorinination of methane 172Ч173
Mechanism chromic acid oxidation 643
Mechanism Claisen condensation 888Ч889
Mechanism Claisen rearrangement 1011Ч1012
Mechanism cyanohydrin formation 719
Mechanism DCCI promoted peptide bond formation 1140
Mechanism decarboxylation of malonic acid 817
Mechanism dehydration of alcohols 206Ч208 221Ч223
Mechanism dehydrohalogenation of alkyl halides 214Ч219 223
Mechanism Dieckmann reaction 890
Mechanism Dieis Ч Alder reaction 409
Mechanism dimerization of 2-methylpropene 266
Mechanism DNA replication 1172Ч1174
Mechanism Edman degradation 1133Ч1135
Mechanism electrophilic addition to alkenes 235Ч242 246
Mechanism electrophilic aromatic substitution 474Ч477 508Ч509
Mechanism electrophilic aromatic substitution bromination, of benzene 481
Mechanism electrophilic aromatic substitution Friedel Ч Crafts acylation, of benzene 485
Mechanism electrophilic aromatic substitution Friedel Ч Crafts alkylation, of benzene 482
Mechanism electrophilic aromatic substitution nitration, of benzene 477Ч478
Mechanism electrophilic aromatic substitution sulfonation, of benzene 478
Mechanism elimination E1 206Ч208 217Ч219
Mechanism elimination E2 206Ч208 214Ч217 223 348Ч350
Mechanism enamine formation 727
Mechanism enol conversion to ketone 380
Mechanism enolization 760 763
Mechanism epoxidation 262
Mechanism epoxide ring opening 680 682
Mechanism esterification 812-813
Mechanism ether cleavage 675
Mechanism ether formation 637
Mechanism free-radical addition of hydrogen bromide to alkenes 242Ч245 274
Mechanism glycosidation 1045
Mechanism halogenation  , of aldehydes and ketones 757Ч761 , of aldehydes and ketones 757Ч761
Mechanism halogenation addition to alkenes 256Ч259 307Ч310
Mechanism halogenation allylic, of alkenes 396Ч398
Mechanism halogenation bromination, of benzene 481
Mechanism halogenation chlorination, of methane 172Ч173
Mechanism halohydrin formation 259Ч260
Mechanism hydration of aldehydes and ketones 716Ч718
Mechanism hydration of alkenes 249
Mechanism hydration of alkynes 380
Mechanism hydride reduction of aldehydes and ketones 628Ч631
Mechanism hydroboration-oxidation 252Ч254
Mechanism hydrogen halide addition to alkenes 235Ч242 297Ч298
Mechanism hydrogenation of alkenes 232
Mechanism hydrolysis of acyl chlorides 840
Mechanism hydrolysis of amides 862Ч867
Mechanism hydrolysis of carboxylic acid anhydrides 844
Mechanism hydrolysis of esters 849Ч852
Mechanism hydrolysis of nitriles 870Ч871
Mechanism hydrolysis saponification 852Ч856
Mechanism imine formation 725
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



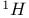 and
and 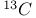
 -keto acids
-keto acids  -unsaturated aldehydes and ketones
-unsaturated aldehydes and ketones  -halogenation of aldehydes and ketones
-halogenation of aldehydes and ketones 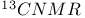 spectrum
spectrum