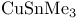|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. Ч Organic Synthesis: Concepts, Methods, Starting materials |

ќбсудите книгу на
Ќашли опечатку?
¬ыделите ее мышкой и нажмите Ctrl+Enter
|
Ќазвание: Organic Synthesis: Concepts, Methods, Starting materials
јвторы: Fuhrhop J., Penzlin G.
язык: 
–убрика: ’ими€/
—татус предметного указател€: √отов указатель с номерами страниц
ed2k:
»здание: second edition, rewise and enlarged
√од издани€: 1994
оличество страниц: 432
ƒобавлена в каталог: 29.10.2005
ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
|
|
 |
| ѕредметный указатель |
 -Muscone = 3-methylcyclopentadecanone 41 -Muscone = 3-methylcyclopentadecanone 41
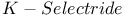 107 107
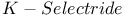 = potassium hydrotris(1-methylpropyl)borate(1-) ( = potassium hydrotris(1-methylpropyl)borate(1-) ( ) 107 ) 107
 107 107
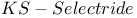 = potassium tris(1,2-dimethylpropyl)hydroborate(1-) ( = potassium tris(1,2-dimethylpropyl)hydroborate(1-) (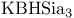 ) 107 ) 107
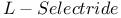 105 107 322Ч323 327Ч328 105 107 322Ч323 327Ч328
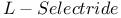 = lithium hydrotris(1-methylpropyl)borate(1-) ( = lithium hydrotris(1-methylpropyl)borate(1-) (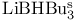 ) 105 107 322Ч323 327Ч328 ) 105 107 322Ч323 327Ч328
 107 107
 = lithium tris(1,2-dimethylpropyl)hydroborate(1-) ( = lithium tris(1,2-dimethylpropyl)hydroborate(1-) (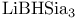 ) 107 ) 107
 -Amino acids, activation 231Ч241 -Amino acids, activation 231Ч241
 -Amino acids, chiral products from 22Ч23 26 61 202Ч203 313Ч314 -Amino acids, chiral products from 22Ч23 26 61 202Ч203 313Ч314
 -Amino acids, pr. 178 184Ч185 -Amino acids, pr. 178 184Ч185
 -Amino acids, protection 228Ч229 -Amino acids, protection 228Ч229
 -Amino acids, stereoselective a-alkylation 299 -Amino acids, stereoselective a-alkylation 299
 -Amino acids, synthesis, -Amino acids, synthesis, 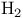 /chiral catalyst 102Ч103 /chiral catalyst 102Ч103
 -Amino acids, synthesis, Strecker synthesis 50 301 -Amino acids, synthesis, Strecker synthesis 50 301
 -D-Glucofuranose, 1,2:5,6-bis-O-(1-methylethylidene) (glucose diacetonide), pr. 263 -D-Glucofuranose, 1,2:5,6-bis-O-(1-methylethylidene) (glucose diacetonide), pr. 263
 -D-Glucofuranose, 1,2:5,6-bis-O-(1-methylethylidene) (glucose diacetonide), syntheses starting from 267 272Ч273 -D-Glucofuranose, 1,2:5,6-bis-O-(1-methylethylidene) (glucose diacetonide), syntheses starting from 267 272Ч273
 -D-Glucofuranose, 1,2:5,6-bis-O-(1-methylethylidene) (glucose diacetonide), synthesis of 267 -D-Glucofuranose, 1,2:5,6-bis-O-(1-methylethylidene) (glucose diacetonide), synthesis of 267
 -Diazo ketones, prepn. and Wolff rearr. 337 -Diazo ketones, prepn. and Wolff rearr. 337
 -hydroxylation of enolates 121Ч122 -hydroxylation of enolates 121Ч122
 -Mannopyranosyl compds., rearrangement to 2-substituted -Mannopyranosyl compds., rearrangement to 2-substituted  -glucopyranosyl fluorides 272 -glucopyranosyl fluorides 272
 -Pinene, (+)-(1R)- and (-)-(1S)-, chiral boranes from 108 -Pinene, (+)-(1R)- and (-)-(1S)-, chiral boranes from 108
 -Pinene, (+)-(1R)- and (-)-(1S)-, pr. 192 -Pinene, (+)-(1R)- and (-)-(1S)-, pr. 192
 -Propoxyphene, (+)- 300 -Propoxyphene, (+)- 300
 -Santalene 42 -Santalene 42
 -Carbolines 292Ч293 -Carbolines 292Ч293
 -Carotene, synthesis 31 41 -Carotene, synthesis 31 41
 -Caryophyllene = [1R-(1R*,4E,9S*)]-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene, synthesis step 78 -Caryophyllene = [1R-(1R*,4E,9S*)]-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene, synthesis step 78
 -Eliminations, C=C double bond formation by, of -Eliminations, C=C double bond formation by, of  -nitro ketones 81 -nitro ketones 81
 -Eliminations, C=C double bond formation by, of alcohols 138 140Ч141 276Ч277 283 -Eliminations, C=C double bond formation by, of alcohols 138 140Ч141 276Ч277 283
 -Eliminations, C=C double bond formation by, of alcohols, exocyclic methylene with -Eliminations, C=C double bond formation by, of alcohols, exocyclic methylene with 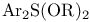 282 282
 -Eliminations, C=C double bond formation by, of alcohols, undesired side-reaction 274 317 -Eliminations, C=C double bond formation by, of alcohols, undesired side-reaction 274 317
 -Eliminations, C=C double bond formation by, of ammonium salts and -Eliminations, C=C double bond formation by, of ammonium salts and  -amino ketones 57 72Ч73 140Ч141 331 -amino ketones 57 72Ч73 140Ч141 331
 -Eliminations, C=C double bond formation by, of epoxides to allylic alcohols 27 -Eliminations, C=C double bond formation by, of epoxides to allylic alcohols 27
 -Eliminations, C=C double bond formation by, of glycosyl halides (HX elimination) 268 -Eliminations, C=C double bond formation by, of glycosyl halides (HX elimination) 268
 -Eliminations, C=C double bond formation by, of halides 52 123Ч124 138 140 283 286 334 -Eliminations, C=C double bond formation by, of halides 52 123Ч124 138 140 283 286 334
 -Eliminations, C=C double bond formation by, of halides from dichloroacetyl chloride 83 276 -Eliminations, C=C double bond formation by, of halides from dichloroacetyl chloride 83 276
 -Eliminations, C=C double bond formation by, of phosphoric 2-(arylsulfonyl)ethyl esters 216Ч217 -Eliminations, C=C double bond formation by, of phosphoric 2-(arylsulfonyl)ethyl esters 216Ч217
 -Eliminations, C=C double bond formation by, of sulfonium salts 38 338 -Eliminations, C=C double bond formation by, of sulfonium salts 38 338
 -Eliminations, C=C double bond formation by, of sulfoxides 65 86 315 -Eliminations, C=C double bond formation by, of sulfoxides 65 86 315
 -Eliminations, oxidative, -Eliminations, oxidative, 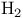 elimination = dehydrogenation 122 138Ч140 249 334 337 338 elimination = dehydrogenation 122 138Ч140 249 334 337 338
 -Eliminations, oxidative, 1,2-bis-decarboxylation 80 138 142 333 337 -Eliminations, oxidative, 1,2-bis-decarboxylation 80 138 142 333 337
 -Eliminations, pyrolytic 138 140Ч141 -Eliminations, pyrolytic 138 140Ч141
 -Eliminations, reductive, of -Eliminations, reductive, of  -acyloxy sulfones 34 -acyloxy sulfones 34
 -Eliminations, reductive, of -Eliminations, reductive, of  -iodo ethers 326Ч327 -iodo ethers 326Ч327
 -Eliminations, reductive, of 1,2-dihalides with iron(0) 78 -Eliminations, reductive, of 1,2-dihalides with iron(0) 78
 -Eliminations, reductive, of 1,2-dihalides with zinc 83 119 156Ч157 -Eliminations, reductive, of 1,2-dihalides with zinc 83 119 156Ч157
 -Eliminations, reductive, of 1,2-diols via cyclic carbonothioates 142 -Eliminations, reductive, of 1,2-diols via cyclic carbonothioates 142
 -Eliminations, reductive, of oxiranes 156Ч157 -Eliminations, reductive, of oxiranes 156Ч157
 -Eliminations, reductive, of tosylhydrazones 14 141Ч142 -Eliminations, reductive, of tosylhydrazones 14 141Ч142
 -Gorgonene 33 -Gorgonene 33
 -Keto carboxylic acids see УCarboxylic acids -Keto carboxylic acids see УCarboxylic acids
 -Lactam antibiotics, synthesis 32 311Ч315 -Lactam antibiotics, synthesis 32 311Ч315
 -Lactams, sensitivity of 311 313 315 -Lactams, sensitivity of 311 313 315
 -Lactams, synthesis from -Lactams, synthesis from  -chloro amides 314 -chloro amides 314
 -Lactams, synthesis from -Lactams, synthesis from  -hydroxv amides (Mitsunohu) 161 -hydroxv amides (Mitsunohu) 161
 -Lactams, synthesis from cyclopropanone + amine 77Ч78 -Lactams, synthesis from cyclopropanone + amine 77Ч78
 -Lactams, synthesis from sulfonyl isocyanate + alkene 153 -Lactams, synthesis from sulfonyl isocyanate + alkene 153
 -Mannopyranosides, special synth. method 271 -Mannopyranosides, special synth. method 271
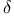 -Lactams by intramol. amidation 291 -Lactams by intramol. amidation 291
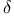 -Lactams by photocyclization 295Ч296 -Lactams by photocyclization 295Ч296
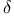 -Lactones, -Lactones,  -vinyl-, Ireland Ч Claisen rearr. 87 -vinyl-, Ireland Ч Claisen rearr. 87
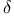 -Lactones, -Lactones, 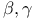 -unsaturated, -unsaturated, 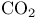 extrusion 80 92 331 extrusion 80 92 331
 -Lactams, -Lactams,  -amino ketone + -amino ketone + 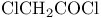 291 291
 -Lactams, 4-oxo acid + amine 314 -Lactams, 4-oxo acid + amine 314
 -Lactams, nitroalkene + -Lactams, nitroalkene + 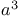 -synthon 65Ч66 112 297 -synthon 65Ч66 112 297
 -Lactones see УCarboxylic acids -Lactones see УCarboxylic acids
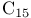 salt 31 salt 31
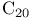 salt 31 salt 31
(+)-DET (diethyl 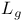 -tartnue), chiral auxiliary 124Ч125 -tartnue), chiral auxiliary 124Ч125
(+)-DET (diethyl 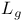 -tartnue), pr. 185 -tartnue), pr. 185
(+)-DIPT (diisopropyt 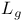 -tartrate), chiral auxiliary 124Ч127 -tartrate), chiral auxiliary 124Ч127
(+)-DIPT (diisopropyt 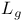 -tartrate), Pr. 185 -tartrate), Pr. 185
(+)-Disparlure, synthesis 125
(+)-DMT (dimethyl 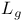 -tartrate), chiral auxiliary 126 -tartrate), chiral auxiliary 126
(+)-DMT (dimethyl 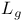 -tartrate), pr. 185 -tartrate), pr. 185
(-)-Limonene see УCyclohexene 1-methyl-4-(1-methylethenyl)-Ф
(4-Methoxyphenyl)mcthyl ethers see УProtectionФ
(l), like-stereoproduct symbol, definition 360
(lk), like-stereoaction symbol, definition 360
(pro-R), stereosymbol, definition 359
(pro-S), stereosymbol, definition 359
(R*), relative configuration stereosymbol 360
(Re), stereoaction symbol, definition 359Ч360
(S*), relative configuration stereosymbol 360
(Si), stereoaction symbol, definition 359Ч360
(Trimethyisilyl)methyl anion see УMagnesium ...Ф
(u), unlike-stereoproduct symbol, definition 360
(ul), unlike-stereoreaction symbol, definition 360
1(2H)-Naphthalenone, 3,4-dihydro- ( -tetralone), 1-spiropentanellation 79 -tetralone), 1-spiropentanellation 79
1(2H)-Pentalenone, hexahydro-, synth. 83
1,1,5,5-Pentanetetracarboxylic acid tetraester 23
1,1-Cyclobutanedicarboxylic acid, diethyl ester 23
1,1-Dicarboxylic acids see УPropanediol acidsФ
1,2-Arenediol derivs., ox. ring opening 87Ч88
1,2-Benzenediol derivs., ozonolysis 87Ч88
1,2-Benziodoxol-3(1H)-one, 1,1,1-tris(acetyloxy)-1,1-dihydro- (Dess Ч Martin periodinane, DMP), oxidation of alcohols 134 328Ч329
1,2-Cyclobutanediol, 1,2-dimethyl-, synthesis 53
1,2-Cyclotetradecancdione, synthesis 54
1,2-Dicarboxylic acids, cyclic, trans-, ring expansion of 53Ч54
1,2-Dicarboxylic acids, ox. decarboxylation 80 142 333 337
1,2-Dicarboxylic acids, red. cyclization 53Ч54
1,2-Dicarboxylic acids, synth. of cyclopentane derivs. from 59 81
1,2-Difunctional compounds 50Ч54 120Ч132
1,2-Difunctional compounds, refro-synthetic analysis 201Ч204
1,2-Diols, conversion to oxiranes 269
1,2-Diols, conversion to oxiranes via chlorohydrin acetates 160 327Ч328
1,2-Diols, ox. cleavage 81Ч82 202 273 292
1,2-Diols, pinacol rearr. of 32
1,2-Diols, reductive  -elimination of 142 -elimination of 142
1,2-Diols, synthesis, opening of oxiranes 117 265 282
1,2-Diols, synthesis, oxidation of alkenes 81Ч82 117 123 127Ч130 265 276Ч277
1,2-Diols, synthesis, oxidation of alkenes, enantioselective 129
1,2-Diols, synthesis, pinacol coupling 53
1,2-Dioxo-compounds, ox. cleavage 137
1,2-Dioxo-compounds, synthesis by  -oxn. of ketones 116 122 137 156 -oxn. of ketones 116 122 137 156
1,2-Dioxo-compounds, synthesis by oxidation of alkynes 117 132
1,2-Dioxo-compounds, synthesis by oxn. of a-hydroxy ketones 133
1,2-Dioxo-compounds, synthesis by Pummerer rearr. 51
1,2-Dioxo-compounds, synthesis via 1-cyclobutene-1,2-diol derivs. 53Ч54
1,2-Ethanediamine, 1,2-diphenyl- (ФstienФ), (R,R)- and (S,S)-, chiral auxiliaries 68Ч69
1,2-Ethanediol, cyclic acetals from 165Ч166 (see also УProtection of carbonyl groupsФ)
1,2-Oxaphosphetane, Wittig transition state 30
1,3,2,4-Dithiadiphosphetane, 2,4-bis(4-methoxyphenyl)- 2,4-disulfide (Lawsson-type reagent), thionation of lactones with 110Ч111
1,3,2-Benzodioxaborole (catecholborane) 131
1,3,2-Diazaborolidines, chiral auxiliaries 68Ч69
1,3,2-Dioxaphosphole, 2,2'-oxybis[4,5-dimethyl-, 2,2'-dioxide, nucleoside coupling with 219
1,3,4-Tfaiadiazoles, 2,5-dihydro-, Barton olelination via 35
1,3,4-Thiadiazolidines, Barton olelination via 35
1,3,5,2,4,6-Trithiatrisihne see УCyclotrisilathianeФ
1,3,5,7,9,11,13,15,17-Cyclooctadecanonaene, (E,E,Z,E,E,Z,E,E,Z)- 40 100
1,3,5,7,9-Cyclodecapentaene isomers 332
1,3,5,7-Cyclooctatetraene, reactions 331Ч333
1,3,5-Benzenetricarboxylic acid (trimesic acid), hydrogenation 347
1,3,5-Cycloheptatrien-1-amine, N-(1-phenylethyl)-7-[(1-phenylethyl)imino]-, (R,R)- (ФchiramtФ), chiral organyl cuprates from 20Ч21
1,3,5-Cycloheptatriene, pr. 191
1,3,5-Cycloheptatriene, synthesis of derivatives 333Ч334
1,3,5-Cyclohexanetricarboxylic acid, 1,3,5-trimethyl-, 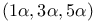 -, derivs. 346Ч348 -, derivs. 346Ч348
1,3,7,9,13,15-Cyclooctadecahexaene-5,11,17-triyne, (E,Z,E,Z,E,Z)- 40 100
1,3-Benzenediols, hydrogenation 87
1,3-Butadiene, 1,1,2,3,4,4-hexachloro, cyclobutenes and squaric acid from 78
1,3-Butadiene, 1,1,2,3,4,4-hexachloro, pr. 186
1,3-Butadiene, 2,3-dimethyl-, diene additions of 85
1,3-Butadiene, 2,3-dimethyl-, pr. 174
1,3-Butadiene, 2-methyl- (isoprene), pr. 174
1,3-Butadiene, 2-methyl- (isoprene), [2+2]cycloaddition 153
1,3-Butadiene, cyclooligomerization 41
1,3-Butadiene, diene additions of 80 81Ч82 85 194 334
1,3-Butadiene, pr. 174
| 1,3-Cyciohexadien-5-yne (ФbenzyneФ), addition to arenes and dienes 92Ч93
1,3-Cyciohexadien-5-yne (ФbenzyneФ), generation of 39 92Ч93
1,3-Cyciohexadien-5-yne (ФbenzyneФ), prepn. in solid Ar matrix 78
1,3-Cyciohexadien-5-yne (ФbenzyneФ), rearr. of thioethers with 39
1,3-Cyclobutadiene 78
1,3-Cyclobutadiene, derivatives, synthesis 329Ч330
1,3-Cyclohexadienes, 5,6-bis(alkylidene)- (o-quinodimechanes) 80 153Ч154 280Ч281 297
1,3-Cyclohexadienes, diene addn. of 85 92 153
1,3-Cyclohexadienes, iron complexes, coupling reactions 44
1,3-Cyclohexadienes, pr. 190
1,3-Cyclohexanediones, 2-alkyl-, Michael addition of 71
1,3-Cyclohexanediones, 2-alkyl-, Robinson anellation of 71 73 212
1,3-Cyclohexanediones, 2-alkyl-, synthesis from 1,3-benzenediol 87
1,3-Cyclopentadiene, 1,2,3,4-tetrachloro-5,5-dimethoxy-, benzo-anellation with 337
1,3-Cyclopentadiene, 5-alkylation 210
1,3-Cyclopentadiene, 5-alkylidene (fulvenes), endoperoxide 102
1,3-Cyclopentadiene, benzvalene from 330
1,3-Cyclopentadiene, diene addns. of 85 92 210 335Ч336
1,3-Cyclopentadiene, diene addns. of, endoperoxide ( ) 276Ч277 ) 276Ч277
1,3-Cyclopentadiene, oxidative dimerization 336
1,3-Cyclopentadiene, pr. 189
1,3-Cyclopentanediones, 2-alkyl-, steroid ring D synthon 71 73 278Ч280
1,3-Cyclopentanediones, 2-alkyl-, synth. 81
1,3-Dicarbonyl type compounds, carbon acidity 9Ч10
1,3-Dicarbonyl type compounds, dianions of 9Ч10 24 204 207 325Ч326
1,3-Dicarboxylic acids, red. cyclization of 53Ч54
1,3-Dienes, cyclic, oxidation to 2-ene-1,4-diols 124 276Ч277
1,3-Dienes, cyclic, oxidation to endo-peroxides 102 276Ч277
1,3-Dienes, cyclic, pr. 189Ч191
1,3-Dienes, cyclic, synth. via 1,2-dihalides 123Ч124 334
1,3-Dienes, derivs. see УDiels Ч Alder type synthesesФ
1,3-Dienes, masked 1,3-diese precursors,  -pyrones 92 331 -pyrones 92 331
1,3-Dienes, masked 1,3-diese precursors, 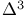 -sulfolenes 153Ч154 -sulfolenes 153Ч154
1,3-Dienes, pr. 174 182Ч183 185Ч186 189Ч191
1,3-Dienes, synthesis via bis(1-alkenyl)boranes 37Ч38
1,3-Difunctional compounds, synthesis 55Ч63 119Ч120
1,3-Dioxanes 165 268Ч269 321Ч323 326 349
1,3-Dioxo compounds, carbon acidity 9Ч10
1,3-Dioxo compounds, cyclic, 1,3-cyclohexanediones 87
1,3-Dioxo compounds, cyclic, 1,3-cyclopentanediones 81
1,3-Dioxo compounds, dianions of 9Ч10 204
1,3-Dioxo compounds, pyrrole synthesis with 150Ч151
1,3-Dioxo compounds, retro-aldol type cleavage of 81 88 155Ч156
1,3-Dioxo compounds, selective reactions with amines 148Ч151 248Ч249 306Ч308
1,3-Dioxo compounds, synthesis by acylation of enamines 88
1,3-Dioxo compounds, synthesis by sulfur extrusion 59Ч60
1,3-Dioxol-2-one, 4,5-diphenyl, УOxФ-protected prim. amines from 164
1,3-Dioxolan-4-ones, 2-alkoxy-2,5,5-trimethyl, protected alcohols 160
1,3-Dioxolane-2-thiones, red. cleavage 142
1,3-Dioxolanes see УProtection of carbonyl groupsФ УProtection
1,3-Dipolar reagents 49 41 152Ч153
1,3-Dithiane, 2-methyl-, acetyl  -synthon 22 51 52 -synthon 22 51 52
1,3-Dithiane, 2-methyl-, synth. from acetaldehyde 22
1,3-Dithiane, formyl  -synthon 17 18 198 267 -synthon 17 18 198 267
1,3-Dithianes 6 8
1,3-Dithianes, hydrolysis 22 51 79 156 267 328Ч329
1,3-Dithianes, reductive desulfurization 109 156
1,3-Dithianes, synthesis from acidic 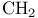 with 1,3-propanedithiol ditosylate 79 156 with 1,3-propanedithiol ditosylate 79 156
1,3-Dithianes, synthesis from aldehydes 8 22 327Ч328
1,3-Dithiolanes, red. desulfurization 109
1,3-Oxathiolanes 165Ч166 (see also УProtectionФ)
1,3-Oxathiolanes, hydrolysis 76 165
1,3-Poylenes, synthesis of, by 1-alkyne coupling 40 155
1,3-Poylenes, synthesis of, by bromination/dehydrobromination 124
1,3-Poylenes, synthesis of, by Heck coupling 42Ч43
1,3-Poylenes, synthesis of, by Julia Ч Lythgoe olefination 34
1,3-Poylenes, synthesis of, by McMurry olefination 41
1,3-Poylenes, synthesis of, by ring opening reactions 87Ч88 332
1,3-Poylenes, synthesis of, by Wittig olefination 30Ч33 281 334
1,3-Propanediamine, monopotassium salt (K 3-aminopropylamtde, KAPA), deprotonation of amines 249Ч250
1,3-Propanediamine, monopotassium salt (K 3-aminopropylamtde, KAPA), deprotonation of carbonyl compounds 10
1,3-Propanediol, 2,2-dimethyl-, pr. 175
1,3-Propanediol, 2,2-dimethyl-, protection of ketones with 165
1,3-Propanediol, 2-amino-1-phenyl-, [S-(R*,R*)]-, chiral 4,5-dihydrooxazoles 22Ч23
1,3-Propanediol, 2-methyl-2-propyl-, pr. 175
1,3-Propanediol, 2-methyl-2-propyl-, synth. and conversion to meprobamate 302
1,3-Propanedithiol, bis(4-methylbenzenesulfonate) ester, 1,3-dithianes from acidic 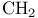 groups 79 156 groups 79 156
1,3-Propanedithiol, preparation of 1,3-dithianes from 8 17 22 328
1,3-PropanediyI, 2-methylene-, e-synthon 84
1,4,10,13-Tetraoxy-7,16-diazacyclooctadecane 247
1,4,2-Dioxazoles 153
1,4,7,10,13,16-hexaoxacyclooctadecane (18-crown-6), phase transfer catalyst 355
1,4-Cycloheptanedione, 2,6,6-trimethyl-, reductive intramolecular coupling 53
1,4-Cycloheptanedione, 2,6,6-trimethyl-, synthesis 76
1,4-Cyclohexadiene-1,2-dicarbonitrile, 4,5-dichloro-3,6-dioxo- (2,3-dichloro-5,6-dicyano-p-quinone, DDQ), oxidations with, of (4-methoxyphenyl)methyl (= MPM = PMB) ethers 157 325 326 329
1,4-Cyclohexadiene-1,2-dicarbonitrile, 4,5-dichloro-3,6-dioxo- (2,3-dichloro-5,6-dicyano-p-quinone, DDQ), oxidations with, of alcohols 133 135 334
1,4-Cyclohexadiene-1,2-dicarbonitrile, 4,5-dichloro-3,6-dioxo- (2,3-dichloro-5,6-dicyano-p-quinone, DDQ), oxidations with, of ketones ( -dehydrogenation) 139Ч140 -dehydrogenation) 139Ч140
1,4-Cyclohexadiene-1,2-dicarbonitrile, 4,5-dichloro-3,6-dioxo- (2,3-dichloro-5,6-dicyano-p-quinone, DDQ), oxidations with, of partially hydrogenated arenes 338
1,4-Cyclohexadiene-1,2-dicarbonitrile, 4,5-dichloro-3,6-dioxo- (2,3-dichloro-5,6-dicyano-p-quinone, DDQ), oxidations with, of porphyrinogens 251 253
1,4-Cyclohexadiens, isomerization and diene addition of 85
1,4-Cyclohexadiens, oxidative ring opening of 87Ч88 206
1,4-Cyclohexadiens, synthesis by Birch reduction of arenes 87 97 103Ч104
1,4-Cyclohexadiens, synthesis by diene addn. to alkynes 194 209Ч210 334 335Ч337
1,4-Dicarboxylic acids, cyclopentane derivs. from 3 55 59 82Ч83
1,4-Dicarboxylic acids, synthesis by ox. opening, from 1, 2-arenediol derivs. 87Ч88
1,4-Dicarboxylic acids, synthesis by ox. opening, from cyclohexanones 82 137
1,4-Difunctional compounds, synthesis 63Ч71 76Ч77 79 90 123Ч124
1,4-Dioxo compounds, 2-cyclopenten-1-ones from 69 79
1,4-Dioxo compounds, heterocycles from 149
1,4-Dioxo compounds, synthesis of 63 65Ч66 69 79
1,4-Epoxynaphthalene, 1,4-dihydro- 93
1,4-Naphthalenedione, 5-hydroxy- (juglone), tetracyclines from 318
1,4:5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a-hexahydro-, 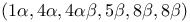 - (isodrin), pagodane and dodecahedrane from 336Ч337 - (isodrin), pagodane and dodecahedrane from 336Ч337
1,4:5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a-hexahydro-, 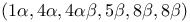 - (isodrin), pr. 192 - (isodrin), pr. 192
1,4a(2H)-Naphthalenediol, octahydro-, isomers, fragmentation 89
1,5-Cyclooctadiene, 9-BBN from 47Ч48
1,5-Cyclooctadiene, oxidative opening 88
1,5-Cyclooctadiene, pr. 191
1,5-Diazabicyclo[4.3.0]non-5-ene = 2,3,4,6,7,8-hexahydropyrrolo[1,2-a]pyrimidine (DBN), HBr elimination with 334
1,5-Dienes see УTerpenes open-chainФ
1,5-Dienes, acid-catd. cyclization of 90Ч92 279Ч280
1,5-Dienes, synth. by allyl coupling 39 41Ч42
1,5-Difunctional compounds, synthesis and reactions 71Ч74 90
1,5-Dioxo compounds, cyclohexenones from 71Ч73
1,5-Dioxo compounds, reductive cyclization of 41
1,5-Dioxo compounds, synthesis of 71Ч73
1,5-Heptadien-4-ol, 3,3,6-trimethyl-, synth. 45
1,5-Hexadiyne, 3-alkylation of 281
1,5-Hexadiyne, ox. cyclooligomerization of 40
1,5-Hexadiyne, [2+2+2]cyclotrimerizations with 80 281
1,5-Polyenes see УTerpenes open-chainФ
1,6-Cyclodecadiene, 1-methyl-, (E,E)- 90
1,6-Dicarbonyl type compounds, synthesis 81Ч82 87Ч88
1,6-Difunctional compounds, synthesis and reactions 81Ч83 87Ч92 136Ч137 206
1,6-Dioxo compounds, synthesis of 87Ч88 136Ч137
1,7,13,19-Cyclotetracosatetraen-3,5,9,11,15,17,21,31-octayne, (all-Z)-, synthesis 155
1-Alken-4-yn-3-ols 303
1-Alkynes, 1-alkoxy-hydration 64Ч65 325 327Ч328
1-Alkynes, 1-halo-, coupling with 1-alkynes 40
1-Alkynes, 1-halo-, hydroboration and coupling 37Ч38
1-Alkynes, acidity 5
1-Alkynes, addition to carbon dioxide 64
1-Alkynes, addition to carbonyl groups 52 62Ч63 278 303
1-Alkynes, addition to carbonyl groups, 2-alkyn-1-ol adducts, pr. 174 182
1-Alkynes, addition to iminium ions 57
1-Alkynes, addition to iminium ions, 2-alkyn-1-amine adducts, pr. 177
1-Alkynes, addition to oxiranes 64Ч65 321
1-Alkynes, hydration to aldehydes 131
1-Alkynes, hydrostannylation with 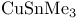 321 321
1-Alkynes, metalation 5
1-Alkynes, metalation with Cu 57
1-Alkynes, metalation with Li or Na 52 64Ч65
1-Alkynes, oxidative coupling 40
1-Alkynes, synthesis, ethynylation of haloalkanes 64
1-Alkynes, synthesis, ethynylation of oxiranes 64 321
1-Alkynes, synthesis, methylidynation of aldehydes 325
1-Azabicyclo[1.1.0]butanes 154
1-Azabicyclo[2.2.2]octane derivs. 309
1-Azabicyclo[2.2.2]octane derivs., pr. 290
1-Azafulvenes 254
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 -Muscone = 3-methylcyclopentadecanone
-Muscone = 3-methylcyclopentadecanone 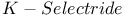
 )
) 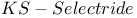
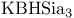 )
) 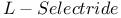
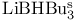 )
) 
 )
)  -Amino acids, activation
-Amino acids, activation  /chiral catalyst
/chiral catalyst  -glucopyranosyl fluorides
-glucopyranosyl fluorides 
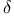 -Lactams by intramol. amidation
-Lactams by intramol. amidation 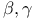 -unsaturated,
-unsaturated, 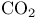 extrusion
extrusion  -Lactams,
-Lactams, 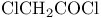
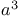 -synthon
-synthon 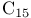 salt
salt 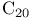 salt
salt 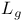 -tartnue), chiral auxiliary
-tartnue), chiral auxiliary 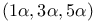 -, derivs.
-, derivs. 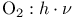 )
) 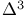 -sulfolenes
-sulfolenes  -synthon
-synthon 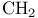 with 1,3-propanedithiol ditosylate
with 1,3-propanedithiol ditosylate  -dehydrogenation)
-dehydrogenation) 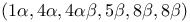 - (isodrin), pagodane and dodecahedrane from
- (isodrin), pagodane and dodecahedrane from