|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. Ч Organic Synthesis: Concepts, Methods, Starting materials |

ќбсудите книгу на
Ќашли опечатку?
¬ыделите ее мышкой и нажмите Ctrl+Enter
|
Ќазвание: Organic Synthesis: Concepts, Methods, Starting materials
јвторы: Fuhrhop J., Penzlin G.
язык: 
–убрика: ’ими€/
—татус предметного указател€: √отов указатель с номерами страниц
ed2k:
»здание: second edition, rewise and enlarged
√од издани€: 1994
оличество страниц: 432
ƒобавлена в каталог: 29.10.2005
ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
|
|
 |
| ѕредметный указатель |
Urea, heterocycles from 148 306
Urea, N-acyl-, undesired by-product 144 234
Uric acid, synthesis 306
Uroporphyrins, synthesis 251Ч252 255
Valium 307
Vesicles, bilayer membrane (BLM) vesicles from lipids or surfactants 350Ч351
Vesicles, covalent 354Ч356
Vesicles, monolayer membrane (MLM) vesicles 351
Vibramycin 317
Vilsmeier reagents (tert. carboxamides + 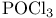 ), ), 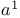 -synthons 16 18 -synthons 16 18
Vilsmeier reagents (tert. carboxamides + 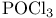 ), alkaloid syntheses via 293 ), alkaloid syntheses via 293
Vilsmeier reagents (tert. carboxamides + 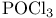 ), formylation of arenes 255 ), formylation of arenes 255
Vincoside (natural alkaloid precursor) 292
Vinyl see УAlkenesФ etc.
Vinylmagnesium bromide see УMagnesium bromoethenyl-Ф
Vinylogous carboxylic acid derivs., vinylogous acids 156 317
Vinylogous carboxylic acid derivs., vinylogous amides 79 249
Vinylogous carboxylic acid derivs., vinylogous thioesters 156
Vinylogous principle 15Ч16 71
Viruses, vehicles for gene transfer 242
Vitamin  , parent system of 259 , parent system of 259
Vitamin 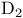 = 9,10-Seco-ergosta-5,7,10(19),22-tetraen-3-ol, = 9,10-Seco-ergosta-5,7,10(19),22-tetraen-3-ol,  - 289 - 289
Vitamin 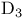 = 9,10-Secocholesta-5,7-10(19)-trien-3-ol, = 9,10-Secocholesta-5,7-10(19)-trien-3-ol,  - 31Ч32 - 31Ч32
Vitamin 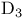 = 9,10-Secocholesta-5,7-10(19)-trien-3-ol, = 9,10-Secocholesta-5,7-10(19)-trien-3-ol,  -, total synthesis 281Ч283 -, total synthesis 281Ч283
Vitamin A see УRetinolФ УCaroteneФ
Vitamin D, provitamins, pr. 284
Vitamin D, provitamins, ring opening 289
Von Braun reaction see УCyanogen bromideФ
Wadsworth Ч Emmons olefination = Horner olefination 28Ч30 32Ч33
Wilkinson type catalysts 97 102Ч103
Wittig and Wittig Ч Horner olefination 28Ч33 (see also УPhosphonium ...Ф)
Wittig and Wittig Ч Horner olefination with phosphine oxides 281
Wittig and Wittig Ч Horner olefination with phosphonic diamides 328Ч329
Wittig and Wittig Ч Horner olefination, cis-selectivity 28Ч31
Wittig and Wittig Ч Horner olefination, sensitivity to steric hindrance 33Ч34
Wolff rearrangement of  -diazo ketones 337 -diazo ketones 337
Wolff Ч Kishner type reduction 97 98 109
Woodward glycolization 128
Woodward Ч Hoffmann rules, examples 262 289
WoodwardТs chlorophyll synthesis 258Ч259
WoodwardТs reagent K 236
Wurtz coupling 36
Xanthates see УCarbonodithioatesФ
Xanthine = 3,7-dthydro-1H-purine-2,6-dione, synthesis and methylation 306
Xanthine alkaloids, caffeine 306
Xanthine alkaloids, pr. 290
Ylenes = Ylides 6Ч7
Ylides see УPhosphoniumФ УSulfoniumФ
Yohimbine, pr. 290
Yohimbine, synthesis steps 292Ч293
Z (benzyloxycarbonyl) see УProtection of aminesФ
Z-Selectivity see Уcis/trans-selectivityФ
| Zinc or zinc-copper, reactions, carbenoid generation from  74Ч75 83 74Ч75 83
Zinc or zinc-copper, reactions, Clemmensen reduction 98 109
Zinc or zinc-copper, reactions, pinacol coupling 53
Zinc or zinc-copper, reactions, reduction of haloalkanes 83 276 335Ч336
Zinc or zinc-copper, reactions, reduction of hydroperoxides 121
Zinc or zinc-copper, reactions, reduction of nitroso groups 268
Zinc or zinc-copper, reactions, reductive  -elimination with, of 1,2-dihalides 119 156Ч157 -elimination with, of 1,2-dihalides 119 156Ч157
Zinc or zinc-copper, reactions, reductive  -elimination with, of glycosyl halides 268 -elimination with, of glycosyl halides 268
Zinc or zinc-copper, reactions, reductive  -elimination with, of oxiranes (deepoxidation) 156Ч157 -elimination with, of oxiranes (deepoxidation) 156Ч157
Zinc or zinc-copper, reactions, reductive  -elimination with, of p-iodo ethers 326Ч327 -elimination with, of p-iodo ethers 326Ч327
Zinc or zinc-copper, reactions, reductive  -elimination with, of trichloroacetyl chloride 83 -elimination with, of trichloroacetyl chloride 83
Zinc or zinc-copper, reactions, reductive cleavage of 2,2,2-trihaloethyl esters, Tbeoc- and Tceoc-alcohols 157Ч158
Zinc or zinc-copper, reactions, reductive cleavage of 2,2,2-trihaloethyl esters, Tbeoc- and Tceoc-amines 162Ч163
Zinc or zinc-copper, reactions, reductive cleavage of 2,2,2-trihaloethyl esters, Tce esters 165
Zinc or zinc-copper, reactions, reductive cleavage of 2,2,2-trihaloethyl esters, Tce phosphates 166Ч167 216 218
Zinc or zinc-copper, reactions, reductive opening of cyclopropanes 76
Zinc or zinc-copper, reactions, Reformatsky reactions with 44Ч45 301
Zinc(2+) acetate, chelation of linear porphyrin precursors 349
Zinc(2+) borohydride see УBorate(1-) tetrahydro- zincФ
Zinc(2+) halides, (1-haloalkyl)cyclopropane opening 69Ч70 77
Zinc(2+) halides, acetalization catalysts 268
Zinc(2+) halides, Mitsunobu reactions with 160Ч161
Zinc, alkylchloro-, alkylation of acyl halides 46
Zinc, diethyl-, Simmons Ч Smith reaction with 75
Zinc, dimethyl-, carbosulfenylation of alkenes 21
Zirconium,  - ( - ( ), hydrozirconation of alkynes with 132 325 ), hydrozirconation of alkynes with 132 325
Zirconium,  -, activation of glycosyl fluorides with -, activation of glycosyl fluorides with  270 270
Zwitterions (betalnes) in Wittig olefination 29Ч30
[10]Annulene, (all-Z)- and (E,Z,E,Z,Z)- 332
[18]Annulene 40 100
[24]Annulene, 1,2,3,4,7,8,9,10,13,14,15,16,19,20,21,22-hexadecadehydro- 155
[5,6]Fullerene- 357 357
УBinapФ, chiral bis-phosphine ligand 102Ч103
УCappingФ of free OH on solid support 221
УCappingФ of non-reacted terminal nucleosides 223
УChiral poolФ, low-cost reagents (ФchironsФ),  -amino acids 178 184Ч185 299 -amino acids 178 184Ч185 299
УChiral poolФ, low-cost reagents (ФchironsФ), alkaloids 290
УChiral poolФ, low-cost reagents (ФchironsФ), hydroxy acids 176 183 185 191
УChiral poolФ, low-cost reagents (ФchironsФ), steroids 284
УChiral poolФ, low-cost reagents (ФchironsФ), sugars 263Ч264
УChiral poolФ, low-cost reagents (ФchironsФ), terpenes 174 182 185 189Ч192 282
УChiramtФ, chiral copper chelates from 20Ч21
УInverted DNAФ 345Ч346
УOxФ protection see УProtection of amino groupsФ
УPicket fenceФ porphyrin 253
УRecombinant bacteriaФ 241Ч246
УRedox glycosidationФ 272
УRФ synthons see УRadical synthonsФ
УSticky endФ method 225Ч226 242Ч244
УStienФ = 1,2-diphenyl-1,2-ethanediamine, (R,R)- and (S,S)-, chiral auxiliaries 68Ч69
УTrimethylenemethaneФ (TMM) synthon 84
УZipФ reaction 249Ч250
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



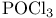 ),
), 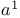 -synthons
-synthons  , parent system of
, parent system of 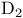 = 9,10-Seco-ergosta-5,7,10(19),22-tetraen-3-ol,
= 9,10-Seco-ergosta-5,7,10(19),22-tetraen-3-ol,  -
- 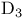 = 9,10-Secocholesta-5,7-10(19)-trien-3-ol,
= 9,10-Secocholesta-5,7-10(19)-trien-3-ol,  -
-  -, total synthesis
-, total synthesis  -diazo ketones
-diazo ketones 
 -elimination with, of 1,2-dihalides
-elimination with, of 1,2-dihalides  - (
- ( ), hydrozirconation of alkynes with
), hydrozirconation of alkynes with  -, activation of glycosyl fluorides with
-, activation of glycosyl fluorides with 
