|
|
 |
| Àâòîðèçàöèÿ |
|
|
 |
| Ïîèñê ïî óêàçàòåëÿì |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. — Organic Synthesis: Concepts, Methods, Starting materials |

Îáñóäèòå êíèãó íà
Íàøëè îïå÷àòêó?
Âûäåëèòå åå ìûøêîé è íàæìèòå Ctrl+Enter
|
Íàçâàíèå: Organic Synthesis: Concepts, Methods, Starting materials
Àâòîðû: Fuhrhop J., Penzlin G.
ßçûê: 
Ðóáðèêà: Õèìèÿ/
Ñòàòóñ ïðåäìåòíîãî óêàçàòåëÿ: Ãîòîâ óêàçàòåëü ñ íîìåðàìè ñòðàíèö
ed2k:
Èçäàíèå: second edition, rewise and enlarged
Ãîä èçäàíèÿ: 1994
Êîëè÷åñòâî ñòðàíèö: 432
Äîáàâëåíà â êàòàëîã: 29.10.2005
Îïåðàöèè: Ïîëîæèòü íà ïîëêó |
Ñêîïèðîâàòü ññûëêó äëÿ ôîðóìà | Ñêîïèðîâàòü ID
|
|
 |
| Ïðåäìåòíûé óêàçàòåëü |
Oligoisoprenes see “Terpenes”
Oligonucleotides, cationic oligonucleotides 343
Oligonucleotides, hexose nucleotides, “homo-DNA” 344—345
Oligonucleotides, syntheses 215—227
Oligonucleotides, syntheses, 1,3,2-dioxaphosphole method 219
Oligonucleotides, syntheses, combined chemical-enzymatic methods 225—227
Oligonucleotides, syntheses, mutagenic modifications 341—342
Oligonucleotides, syntheses, phosphite methods 219—224 341—343
Oligonucleotides, syntheses, phosphodiester method 216—217
Oligonucleotides, syntheses, phosphotriester method 217—218
Oligonucleotides, syntheses, solid-phase methods 221—224 341—343
Oligonucleotides, syntheses, “sticky end” method 225—226
Oligopeptides see “Peptides”
Oligosaccharides, syntheses 269—272
Oppenauer oxidation 133—134 278 285
Oppenauer oxidation, epimerization of alcohols by 288
Optical resolution in drug synth. 300—301 309
Optical resolution in natural product synthesis 319—320 327
Optical resolution, enantioselective synthesis, “kinetic resolution”, enzymatic 167 277 278
Optical resolution, enantioselective synthesis, “kinetic resolution”, Noyori hydrogenation 102—103 325—326
Optical resolution, enantioselective synthesis, “kinetic resolution”, Sharpless epoxidation 124—127 264—265 321—325
Organometallic compounds see “1-Alkynes” or the corresponding metals or metal complexes
Organometallic compounds, metal-metal exchange 5 7
Organometallic compounds, metal-metal exchange with copper(1+) 5 20—21
Organometallic compounds, metal-metal exchange with zinc(2+) 5 46
Organometallic compounds, organyl coupling reactions 36 40—44
Organometallic compounds, preparation, from C-H-acidic compounds 5—9
Organometallic compounds, preparation, from haloalkanes, with alkylUthium 70
Organometallic compounds, preparation, with metals 5—7 17 20 46
Organometallic compounds, reactivity, effect of metal counterions 5 40
ortho-Quinodimethanes = 5,6-bis(alkylidene)-1,3-cyclohexadienes 280—281 297
Orthoformic acid, esters (trimethoxymethane and 1,1',1''-[methylidynetris(oxy)]tris[ethane]), formyl 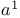 -synthon 18 58—59 198 -synthon 18 58—59 198
Orthoformic acid, esters (trimethoxymethane and 1,1',1''-[methylidynetris(oxy)]tris[ethane]), Meerwein’s dialkoxymethylium reagents 38 58—59
Orthoformic amides see “Methanamine ...”
Orthoformic diamides see “Methanediamine ...”
Osmium(8+) oxide (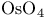 or or 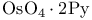 ), oxidation of alkenes to 1, 2-diols 81—82 128—129 210 282 ), oxidation of alkenes to 1, 2-diols 81—82 128—129 210 282
Osmium(8+) oxide (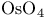 or or 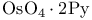 ), oxidation of alkenes to 1, 2-diols, ), oxidation of alkenes to 1, 2-diols, 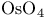 -catalysed 82 129 282 -catalysed 82 129 282
Osmium(8+) oxide (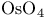 or or 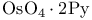 ), oxidation of alkenes to 1, 2-diols, enantioselective 129 ), oxidation of alkenes to 1, 2-diols, enantioselective 129
Osmium(8+) oxide (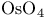 or or 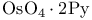 ), toxicity 128 ), toxicity 128
Oxa-Cope (= Claisen) rearrangement 87
Oxacyclotrideca-5,9-dien-2-one, (E,E)- 42
Oxalyl chloride see “Ethanedioyl dichloride”
Oxazoies, 2-aikyl-4,5-dihydro-,  -alkylation 13 22—23 -alkylation 13 22—23
Oxazoies, 2-aikyl-4,5-dihydro-, enantioselective syntheses with 22—23
Oxazoies, 2-aikyl-4,5-dihydro-, preparation 22—23 153
Oxidation 115—140 (see also “Eliminations oxidative” “Rearrangements oxidative” “Corresponding
Oxidation of 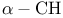 of ketones 79 121—122 156 of ketones 79 121—122 156
Oxidation of alcohols 133—136
Oxidation of alkanes 115—116 118—119 285—286
Oxidation of alkenes 116—117 119—121 123—131
Oxidation of alkenes to 1,2-dihalides 84 118—119 123—124 156—157
Oxidation of alkenes to 1,2-diols 81—82 117 123 127—130 265 276—277
Oxidation of alkenes to 1,2-diols, enantioselective 129
Oxidation of alkenes to haloethers 272 326
Oxidation of alkenes to halohydrins 77 117 275—276 287
Oxidation of alkenes to halolactones 275 319—321 335—336
Oxidation of alkenes to oxiranes (= epoxides) 117
Oxidation of alkenes to oxiranes (= epoxides) by enantioselective Sharpless oxidation 124—127 265 320—325
Oxidation of alkenes to oxiranes (= epoxides) by hydrogen peroxide (2-enones) 275
Oxidation of alkenes to oxiranes (= epoxides) by peracids 117 123—124 156—157
Oxidation of alkenes to oxiranes (= epoxides) via halohydrinations 77 117 287 319—320 327—328
Oxidation of alkenes, allylic oxidation 119—121
Oxidation of alkylarenes 120
Oxidation of alkylboranes with N-oxidcs 37
Oxidation of alkynes 117 131—132
Oxidation of dienes to 2-ene-1,4-diols 124 276—277
Oxidation of hydrazines to diazenes 35 331
Oxidation of phosphines to P-oxides 282
Oxidation of phosphite esters 219—220 223
Oxidation of tert. amines 116 120—121 138—139
Oxidation, remote oxidation 116 118 285—286
Oxidation, table 116—118
Oxidative cleavage, degradation or rearrangement of  -amino acids 48 -amino acids 48
Oxidative cleavage, degradation or rearrangement of 1,2-arenediol derivatives 87—88
Oxidative cleavage, degradation or rearrangement of 1,2-diols 81—82 88 202 273 292
Oxidative cleavage, degradation or rearrangement of 2-enones 82
Oxidative cleavage, degradation or rearrangement of alkenes 81—82 87—88 202 259 280
Oxidative cleavage, degradation or rearrangement of alkynes 117 132
Oxidative cleavage, degradation or rearrangement of arylsilanes with 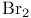 80 80
Oxidative cleavage, degradation or rearrangement of ketones see “Ketones”
Oxidative cleavage, degradation or rearrangement of oxiranes 88
Oxidative cleavage, degradation or rearrangement of p-methoxybenzyl ethers 157 325—329
Oxidative cleavage, degradation or rearrangement of tert. amines with BrCN 309
Oxidative cleavage, degradation or rearrangement of thioethers 314—315
Oxidative cleavage, degradation or rearrangement of triorganylboranes 47—48 130—131
Oxidative cleavage, degradation or rearrangement of “Ox” protecting groups 164
Oxidative coupling in 1-alkenyl boranes 37—38
Oxidative coupling of 1-alkynes 40 154—155 339
Oxidative coupling of enol silyl ethers 65
Oxidative coupling of phenols 293—295
Oxidative decarboxylations see “Decarboxylation”
Oxidative p-elimination see “ -Elimination” -Elimination”
Oxidative sulfitolysis 239—240
Oximes, Beckmann rearr. 136—137
Oximes, heterocycles from 307—308
Oximes, reduction of 98 112
Oxirane (ethylene oxide), 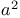 -synthon 64 149 198 281 300 -synthon 64 149 198 281 300
Oxirane (ethylene oxide), 2, 3-dimethyl-, (R,R)-, synthesis with 64
Oxirane (ethylene oxide), 2-decyl-3-(5-methylhexyl)-, cis- (gipsy moth pheromone), syntheses 34 125
Oxirane (ethylene oxide), 2-ethyl-, alkynylation 64
Oxirane (ethylene oxide), 2-ethyl-, pr. 175
Oxirane (ethylene oxide), 2-methyl- (propylene oxide), alkylation with 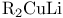 21 21
Oxirane (ethylene oxide), 2-methyl- (propylene oxide), pr. 175
Oxiranes from  -halo ketones by enantioselective reduction 108 -halo ketones by enantioselective reduction 108
Oxiranes from 1,2-diols 160 269 327—328
Oxiranes from 2-enones with 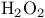 274—275 274—275
Oxiranes from carbonyl compounds 45
Oxiranes from halohydrins 77 117 160 287 319—320 327—328
Oxiranes, 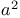 -synthons 16 44 63—65 -synthons 16 44 63—65
Oxiranes, opening with 1-alkynes 64—65 321 325 327—328
Oxiranes, opening with acetate/acetic acid 282
Oxiranes, opening with acids 322
Oxiranes, opening with amines 291
Oxiranes, opening with benzenethiol 265
Oxiranes, opening with carboxylic acid dianions 63
Oxiranes, opening with diorganylcuprates(1-) 21 44 269
Oxiranes, opening with hydrogen fluoride 287
Oxiranes, opening with hydroxide 123—124 265
Oxiranes, opening with imidic ester ec-anions 63—64
Oxiranes, pr. 175
Oxiranes, red. opening 98 114 274—275 319—320
Oxiranes, synthesis from alkenes by Sharpless epoxidation 124—127 265 320—325
Oxiranes, synthesis from alkenes via halohydrinations 77 117 287 319—320 327—328
Oxiranes, synthesis from alkenes with hydrogen peroxide 274—275
Oxiranes, synthesis from alkenes with peracids 117 123—124 156—157
Oxonium, (methoxymethylene)methyl- see “Methylium dimethoxy-”
Oxonium, triethyl-, O-alkylation of carboxamides 111—112 260
Oxonium, triethyl-, O-alkylation of vinylogous carboxamides 249
Oxonium, triethyl-, S-alkylation of thioethers 39
Oxonium, trimethyl-, methyl  -synthon 16 -synthon 16
Oxygen, oxn. of organic anions 19 121
Oxygen, singlet-state, oxidative cleavage of alkenes with 259
Oxygen, singlet-state, [2+4]cycloaddition of 102 276—277
Oxymetazoline 305
Oxyphenbutazone,  - 305 - 305
Oxytetracycline,  -, total synthesis 318 -, total synthesis 318
Ozonolysis of 1,2-arenediol derivatives 87—88
Ozonolysis of alkenes 87—88 156—157 280 285 322 323 326
Ozonolysis of enamines 285
Ozonolysis of enol esters and ethers 87—88 280
Ozonolysis of hydrazones 26
Pagodane, rearr. to dodecahedrane 336
Pagodane, synthesis 336—337
Palladium see “Hydrogenation” “Hydrogenolysis”
Palladium(0) complexes,  -allyl-Pd(0), allylation with 27 164 303 -allyl-Pd(0), allylation with 27 164 303
Palladium(0) complexes,  -allyl-Pd(0), polymer-bound stereoselective 164 303 -allyl-Pd(0), polymer-bound stereoselective 164 303
Palladium(0) complexes, catalysts for 1,5-enyne cycloisomerization 84
Palladium(0) complexes, catalysts for coupling reactions 42 295
Palladium(0) complexes, catalysts for trimethylenemethane generation 84
Palladium(2+),  -allyl complexes from 27 -allyl complexes from 27
| Palladium(2+), catalysts for Heck coupling 42—43
Palladium(2+), catalysts for hydrogen 1,3-shifts 90
Palladium, exhaustive dehydrogenation 139—140 337
Panizzi formylation 58—59
Papaverine alkaloids, conversions 294—295
Papaverine alkaloids, pr. 290
Papaverine alkaloids, syntheses 293
para-Cyclophanes = ![$tricyclo[8.2.2.2^{4,7}]hexadeca-4,6,10,12,13,15-hexaenes$](/math_tex/dff428664ed8b0b8d75ae2c5ac3fa75282.gif) , synth. 38—39 , synth. 38—39
PCC see “Chromate(1-) chlorotrioxo- pyridinium”
PCR = polymerase chain reaction 226—227
Pechmann’s 1,2,3-triazole synthesis 151—152
Penam 311
Penem derivative 32
Penicillanic acid, 6-amino- (6-APA) 311—312
Penicillin derivs., conversion to cephalexins 315
Penicillin derivs., prepn. from penicillin G 311—312
Penicillin derivs., syntheses 32 313—314
Pentanoic acid, 2-ethyl-, retro-synthesis 199—201
Pentanoic acid, 4-oxo- (levulinic acid), cephalosporins from 313—314
Pentanoic acid, 4-oxo- (levulinic acid), indole synthesis from 307
Pentanoic acid, 4-oxo- (levulinic acid), pr. 177
Pentanoic acid, 4-oxo- (levulinic acid), retro-synthesis with 205
Pentanoic acid, 5-bromo-, esters, 5-acylation 47
Pentanoic acid, 5-bromo-, esters, pr. 180
Pentazocine, (-)-, synthesis 309
Pentonic acids, esters, 2-hexulosides from 272
Peptides, activation of carboxylate 143—146 230—241
Peptides, genetic engineering methods 241—246
Peptides, protection of functional groups 161—165 229
Peptides, racemization, prevention of 145 231—232
Peptides, solid-phase methods 232—237 241
Peptides, solution (= liquid-phase) methods 237—241
Peptides, synthesis 228—246
Peracetic acids see “Ethaneperoxoic acids”
Peracids see “Carboperoxoic acids”
Perbenzoic acids see “Benzenecarboperoxoic acids”
Perchloric acid Li salt Diels—Alder 86
peri-Substituents, crowding of see “Steric strain”
Pericyclic reactions see “ -Eliminations pyrolytic” -Eliminations pyrolytic”
Pericyclic reactions, aldol type reactions 59—62
Pericyclic reactions, carbonyl allylations 67—69
Pericyclic reactions, hydride shift from alkylborane to ketone 108
Pericyclic reactions, Moflatt — Pfitzner oxn. 133
Pericyclic reactions, oxidations with 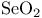 119 122 119 122
Pericyclic reactions, Reformatsky reactions 44—45
Periodate (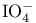 ) or periodic acid ( ) or periodic acid ( ), oxidation of phenylselenyl groups 139 ), oxidation of phenylselenyl groups 139
Periodate (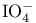 ) or periodic acid ( ) or periodic acid ( ), oxidative cleavage of ), oxidative cleavage of  -hydroxy ketones 62 -hydroxy ketones 62
Periodate (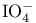 ) or periodic acid ( ) or periodic acid ( ), oxidative cleavage of 1,2-diols 81—82 272—273 282 292 ), oxidative cleavage of 1,2-diols 81—82 272—273 282 292
Periodate (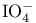 ) or periodic acid ( ) or periodic acid ( ), oxidative cleavage of 1,2-diones 137 ), oxidative cleavage of 1,2-diones 137
Periodate (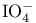 ) or periodic acid ( ) or periodic acid ( ), oxidative cleavage of oxiranes 88 ), oxidative cleavage of oxiranes 88
Periodinane reagent, Dess — Martin 134 328—329
Permanganate, potassium, ox. cleavage of ketones 137
Permanganate, potassium, oxidation with, of  -CH of carboxylic acids 120—121 -CH of carboxylic acids 120—121
Permanganate, potassium, oxidation with, of alkenes 128
Permanganate, potassium, oxidation with, of alkynes 117 132
Permanganate, potassium, oxidation with, of benzylic CH 120
Peroxide, hydrpgen see “Hydrogen peroxide”
Peroxymolybdenum complex see “Molybdenum”
Peterson olefination 28 33—34
PG see “Prostaglandins”
Phase-transfer catalysts see “Benzenemethanaminium ...” “Butanaminium ...” “18-Crown-6”
Phenazopyridine 306
Phenols via ortho-alkylation and rearr. 319—320
Phenols, derivatives, O-arlyation (copper-catalysed) 295
Phenols, derivatives, oxidative C-C and C-N coupling 293—294
Phenols, derivatives, palladation and coupling reactions 42—43
Phenols, para-alkylation of 93
Phenothiazines, synthesis 309—310
Phenyl see “Benzene ...”
Phenylalanine, N-acyl-D- or -L-, synth. 102—103
Phenylbutazone 305
Phenylephrine, (-)-(R)- 301
Phenylpropanolamine,  - 302 - 302
Pheromones, synth., by Peterson olefination 34
Pheromones, synth., by Wiuig olefination 31
Phlorins 258
Phosphate esters see “Phosphoric acid esters”
Phosphine oxides, deoxygenation with 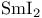 115 115
Phosphine oxides, synthesis, alkyl halide + 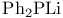 282 282
Phosphine oxides, Wittig — Horner type olefination with 281
Phosphine, 1,2-ethanediylbis[diphenyl- (Diphos), chelation of Pd(2+) 27
Phosphine, diphenyl-, Li salt, 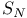 with 282 with 282
Phosphine, tributyl-, stabilization of organylcopper 277
Phosphine, triphenyl-, P-aikylation of 17 32
Phosphine, triphenyl-, palladium complex catalysts 27 42 295
Phosphine, triphenyl-, reagent with iodine, OH/I exchange 328
Phosphine, triphenyl-, reagents with diazenedicarboxylic esters, Mitsunobu reactions with 160—161 286
Phosphine, triphenyl-, reagents with N-halosuccinimides, conversion of alcohols to halides 169
Phosphine, triphenyl-, reagents with tetrahalomethanes, conversion of prim, alcohols to halides 266
Phosphine, triphenyl-, sulfur extrusion with, from disulfides 39
Phosphine, triphenyl-, sulfur extrusion with, from thiiranes 35 59—60 334
Phosphine, triphenyl-, thioesters from disulfides 146 319—320
Phosphine, [1,1'-binaphthalene]-2,2'-diylbis[diphenyl- (Binap), (R)- and (S)-, chiral Noyori hydrogenation catalysts from 102—103
Phosphite esters see “Phosphorous acid esters”
Phospholipid membranes 350
Phosphonic acid, (diazomethyl)-, dialkyl ester, methylidynation of aldehydes to 1-alkynes 325
Phosphonic acid, 1,3-propanediylbis[-, cyclizn. with 334
Phosphonic acid, alkyl-, dialkyl esters, Horner olefinations by 29 30 267 272—273
Phosphonic acid, alkyl-, dialkyl esters, pr. 188
Phosphonic acid, alkyl-, dialkyl esters, relative reactivity 28—29
Phosphonic acid, diesters, oxidative amidation to phosphoramidates 343
Phosphonic diamide, P-ethyl-N,N,N',N'-tetramethyl-, alkylation and olefination 327—329
Phosphonium ylides 6—7 28—32
Phosphonium ylides, olefination with see “Phosphonium ...”
Phosphonium ylides, olefination with, relative reactivities 28—29
Phosphonium ylides, olefination with, Z-selectivity and mechanism 28—31
Phosphonium ylides, reaction with triorganylboranes 9
Phosphonium, (1,3-propanediyl)bis[triphenyl-, cyclizations with 334
Phosphonium, (1-methylethyl)triphenyl- 283 322
Phosphonium, (2-methoxy-2-oxoethyl)triphenyl-, pr. 188
Phosphonium, (2-methoxy-2-oxoethyl)triphenyl-, Wittig reaction 32—33
Phosphonium, (2-oxoethyl)triphenyl-, monosaccharide homologization by 264—265
Phosphonium, (2-oxoethyl)triphenyl-, pr. 188
Phosphonium, (4-carboxybutyl)triphenyl-, pr. 188
Phosphonium, (4-carboxybutyl)triphenyl-, prostaglandin synthon 7 272—273 275—276
Phosphonium, (alkoxymethyl)triphenyl-, formyl  -synthons 6—7 18 48—49 -synthons 6—7 18 48—49
Phosphonium, (alkoxymethyl)triphenyl-, pr. 188
Phosphonium, alkyltriphenyl-, alkylidene d-synthons 7 31—32 201 203
Phosphonium, alkyltriphenyl-, pr. 188
Phosphonium, ethenyltriphenyl-, diene additions to 85
Phosphonium, ethyltriphenyl-, pr. 188
Phosphonium, ethyltriphenyl-, Wittig reaction 283
Phosphonium, hexyltriphenyl, pr. 188
Phosphonium, hexyltriphenyl, Wittig reaction 321
Phosphonium, methyltriphenyl-, pr. 188
Phosphonium, methyltriphenyl-, Wittig reaction 31—32
Phosphonium, pentyltriphenyl-, pr. 188
Phosphonium, pentyltriphenyl-, Wittig reaction 31
Phosphonium, triphenyl[1-(phenylthio)cyclopropyl]-, 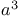 -synthon for cyclopentanellations 16 83 -synthon for cyclopentanellations 16 83
Phosphonium, [3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenyl]triphenyl-, (all-E)- (”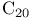 salt”) 31 salt”) 31
Phosphonium, [3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienyl]triphenyl-, (E,E) 31
Phosphonium, [thiobis(methylene)]bis[triphenyl-, cyclizations with 334
Phosphoramidate-linked oligonucleotides 343
Phosphoramidite method, oligonucleotide synthesis 220—224 341—343
Phosphorane, pentachloro- see “Phosphorus(5+) chloride”
Phosphoranes, (alkylideneazino)- see “Ketones (triphenylphosphoranylidene)hydrazones”
Phosphoranes, alkylidene- see “Phosphonium ...”
Phosphoric acid, 4-chlorophenyl ester, nucleoside coupling with 217—218
Phosphoric acid, esters see “Nucleotides”
Phosphoric acid, esters, prepn. by Mitsunobu type reaction 160—161
Phosphoric triamide, hexamethyl- (HMPTA, HMPA) in Mimoun’s Mo complex 121—122
Phosphoric triamide, hexamethyl- (HMPTA, HMPA), reducing agent  115 115
Phosphorodichloridous acid, 2,2,2-trichloroethyl ester, nucleoside coupling with 220
Phosphorous acid, esters, Mitsunobu type reactions with 160—161
Phosphorous acid, esters, oxidation of 219—220 223 342 343
Phosphorous acid, esters, red. cleavage of 1,3-dioxolane-2-thiones 142
Phosphorous acid, esters, reduction with, of 1,2-dione via 1,3,2-dioxaphosphole 219
Phosphorous acid, esters, reduction with, of hydroperoxides 121
Phosphorous acid, esters, sulfur extrusion from thiiranes 35 59—60 261
Phosphorous acid, esters, sulfur extrusion from thioethers by UV irradiation 39
|
|
 |
| Ðåêëàìà |
 |
|
|
 |
|
Î ïðîåêòå
|
|
Î ïðîåêòå



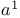 -synthon
-synthon 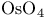 or
or 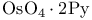 ), oxidation of alkenes to 1, 2-diols
), oxidation of alkenes to 1, 2-diols  -alkylation
-alkylation 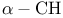 of ketones
of ketones 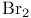
 -Elimination”
-Elimination”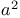 -synthon
-synthon 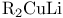
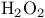
 -
-  -allyl-Pd(0), allylation with
-allyl-Pd(0), allylation with ![$tricyclo[8.2.2.2^{4,7}]hexadeca-4,6,10,12,13,15-hexaenes$](/math_tex/dff428664ed8b0b8d75ae2c5ac3fa75282.gif) , synth.
, synth. 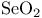
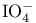 ) or periodic acid (
) or periodic acid ( ), oxidation of phenylselenyl groups
), oxidation of phenylselenyl groups 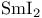
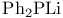
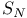 with
with  -synthons
-synthons 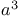 -synthon for cyclopentanellations
-synthon for cyclopentanellations  salt”)
salt”) 