|
|
 |
| Àâòîðèçàöèÿ |
|
|
 |
| Ïîèñê ïî óêàçàòåëÿì |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. — Organic Synthesis: Concepts, Methods, Starting materials |

Îáñóäèòå êíèãó íà
Íàøëè îïå÷àòêó?
Âûäåëèòå åå ìûøêîé è íàæìèòå Ctrl+Enter
|
Íàçâàíèå: Organic Synthesis: Concepts, Methods, Starting materials
Àâòîðû: Fuhrhop J., Penzlin G.
ßçûê: 
Ðóáðèêà: Õèìèÿ/
Ñòàòóñ ïðåäìåòíîãî óêàçàòåëÿ: Ãîòîâ óêàçàòåëü ñ íîìåðàìè ñòðàíèö
ed2k:
Èçäàíèå: second edition, rewise and enlarged
Ãîä èçäàíèÿ: 1994
Êîëè÷åñòâî ñòðàíèö: 432
Äîáàâëåíà â êàòàëîã: 29.10.2005
Îïåðàöèè: Ïîëîæèòü íà ïîëêó |
Ñêîïèðîâàòü ññûëêó äëÿ ôîðóìà | Ñêîïèðîâàòü ID
|
|
 |
| Ïðåäìåòíûé óêàçàòåëü |
Barbiturates 306
Barrelene = bicyclo[2.2.2]octa-2,5,7-triene 331
Barton olefination 35
Barton oxidation of alkyl groups 286
Bases for enolate generation 10—11
Bases, strong ( ) see “Amides” “Ethanol “Lithium butyl-” “Lithium methyl-” “Lithium (1-methylpropyl)-” “Magnesium bromoethyl-” “Methane sulfinyl-bis[- ion(1-)” “Methanol “1 K ) see “Amides” “Ethanol “Lithium butyl-” “Lithium methyl-” “Lithium (1-methylpropyl)-” “Magnesium bromoethyl-” “Methane sulfinyl-bis[- ion(1-)” “Methanol “1 K
Bases, strong bulky see “1-Butanol 3-methyl-1 Li “2-Butanol 2-methyl- ion(1-)” “Lithium (1 “Lithium (triphenylmethyl)-” “Lithium-naphthalene “Potassium (triphenylmethyl)- “Potassium-naphthalene “2-Propanamine N-(1-methylethyl)- lithium “2-Propanol 2-methyl- ion(1-) “Silanamine 1 Na
Bases, strong solid-phase see “Hydrides alkali”
Bases, weak bulky (non-nucleophilic) see “1 (DBN)” “Ethanamine N ( “1-Naphlhalenamine N (Huenig “2-Propanamine N-ethyl-N-(1-methylethyl)- “Pyridine 2 “Pyridine 2 “Silanamine 1 (HMDS)”
Bases, weak solid-phase see “Aluminum(3+) oxide” “Carbonate calcium” “Fluoride potassium “Huenig polymeric” “Sodium-sand
Bases, “weak” ( ) see “Acetate piperidinium” “Benzenemethanaminium N hydroxide “Carbonates alkali” “Carbonates alkali “Hydroxides alkali” “Lead(2+) basic Pyridine” ) see “Acetate piperidinium” “Benzenemethanaminium N hydroxide “Carbonates alkali” “Carbonates alkali “Hydroxides alkali” “Lead(2+) basic Pyridine”
Basketene = ![$pentacyclo[4.4.0.0^{2,5}.03^{3,8}.0^{4,7}]dec-9-ene$](/math_tex/579817118bbad24592ab7a04af75574882.gif) , photo dimer 335 , photo dimer 335
Basketene = ![$pentacyclo[4.4.0.0^{2,5}.03^{3,8}.0^{4,7}]dec-9-ene$](/math_tex/579817118bbad24592ab7a04af75574882.gif) , synthesis steps 142 333 , synthesis steps 142 333
Beckmann rearrangement 136—137
Benzene, (bromomethyl)- (benzyl bromide), O-protection 157 229
Benzene, (chloromethyl)- (benzyl chloride), O-protection 157 158—159
Benzene, 1-(bromomethyl)-4-methoxy- (pMbBr), O-protection 157 325
Benzene, 1-bromo-2-fluoro-, benzyne from 39
Benzene, 1-methoxy-4-methyl-, ozonolysis 88
Benzene, 1-methoxy-4-methyl-, reduction 87
Benzene, 1-[(isocyanomethyl)sulfonyl]-4-methyl-(TosMIC), synthesis with, of  -hydroxy-aldehydes 49 51 -hydroxy-aldehydes 49 51
Benzene, 1-[(isocyanomethyl)sulfonyl]-4-methyl-(TosMIC), synthesis with, of nitriles 49
Benzene, ethenyl- (styrene), (S)-1-oxiranylated polymer 108
Benzene, ethenyl- (styrene), chloromethylated aminated homopolymer (polymeric Huenig base), deprotonation with 11 32
Benzene, ethenyl- (styrene), chloromethylated aminated homopolymer (polymeric Huenig base), removal of acids with 32
Benzene, ethenyl- (styrene), chloromethylated copolymer with diethenyl- benzenes, peptide synth. with 232—236
Benzene, ethenyl- (styrene), chloromethylated diphenylphosphinated polymer, solid Pd complex catalyst 164
Benzene, ethenyl- (styrene), sulfonated homopolymer, acid catalyst for acetai hydrolysis 267
Benzene, ethenyl- (styrene), sulfonated homopolymer, acid catalyst for aldol addition 55
Benzene, ethenyl- (styrene), sulfonated homopolymer, acid catalyst for ester solvolysis 282
Benzene, hexamethyl-, synthesis 330
Benzeneacetaldehyde, enolate addition to 58
Benzenecarboperoxoic acid, 2-carboxy-, epoxidation with 123—124
Benzenecarboperoxoic acid, 3-chloro- (MCPBA), Baeyer — Villiger oxidation with 165 283
Benzenecarboperoxoic acid, 3-chloro- (MCPBA), epoxidation with 27 123—124
Benzenecarboperoxoic acid, 3-chloro- (MCPBA), ox. cleavage of “Ox”-protected amines 164
Benzenecarboperoxoic acid, 3-chloro- (MCPBA), oxidation of thioethers with 265 315
Benzenecarboperoxoic acid, epoxn. with 123—124
Benzenemethanaminium, N,N,N-trimethyl-, hydroxide (Triton B), deprotonation with, of nitriles 304
Benzenemethanaminium, N,N,N-trimethyl-, hydroxide (Triton B), deprotonation with, of nitroalkanes 66
Benzenemethanol,  -(1-aminoethyl)-, (S,R)-((+)-norephedrine), chiral auxiliary 61 -(1-aminoethyl)-, (S,R)-((+)-norephedrine), chiral auxiliary 61
Benzeneselenenyl bromide, dehydrogenation with 138 139
Benzenesuifonyl azide, 4-methyl- ( ), prepn. of ), prepn. of  -diazo ketones from ketones 337 -diazo ketones from ketones 337
Benzenesuifonyl chloride, 2,4,6-trimethyl- (2-mesitylenesulfonyl chloride), nucleotide coupling with 218
Benzenesuifonyl chloride, 2,4,6-tris(1-methylethyl)- (TpsCl), nucleotide coupling with 217
Benzenesuifonyl chloride, 4-amino-, drugs from 307—308
Benzenesuifonyl chloride, 4-methyi-, amides from 69
Benzenesuifonyl chloride, 4-methyi-, tosylate esters from 269 281 291
Benzenesuifonyl triazolides, nucleotide coupling with 218
Benzenesulfenyl chlorides + ![$\mathrm{Me_{2}Zn[TiCl_{4}]}$](/math_tex/0f70b1c11e62aee366c0b1ebc400f2b582.gif) , Reetz carbosulfenylation of alkenes 21 , Reetz carbosulfenylation of alkenes 21
Benzenesulfonamide, N,N-dibromo-4-methyl- ( ), bromohydrination with 287 ), bromohydrination with 287
Benzenesulfonamides, reductive cleavage 247
Benzenesulfonamides, synthesis of drugs 301 307—308
Benzenesulfonic acid, 4-methyl-, esteis (tosylates), alkyl a-synthons 16 24 47 93
Benzenesulfonic acid, 4-methyl-, esteis (tosylates), fragmentation of 89
Benzenesulfonic acid, 4-methyl-, esteis (tosylates), rearrangement of 32
Benzenesulfonic acid, 4-methyl-, esteis (tosylates), reductive cleavage of 114 202—203
Benzenesulfonic acid, 4-methyl-, hydrazide (tosylhydrazine), hydrazones of, base-catd. elimn. or rearr. 141—142
Benzenesulfonic acid, 4-methyl-, hydrazide (tosylhydrazine), hydrazones of, fragmentation 89
Benzenesulfonic acid, 4-methyl-, hydrazide (tosylhydrazine), hydrazones of, reduction 109
Benzenesulfonic acids see “Sulfonic acids”
Benzenesulfonothioic acid, 4-methyl-, S,S'-(1,3-propanediyl) ester see “Propane-1 ...”
Benzenethiol, cleavage of methyl phosphates 224
Benzenethiol, opening of oxiranes 265
Benzo-anellation procedure 337
Benzocyclobutenes see “Bicyclo[4.2.0]octa-1
Benzoic acid, 2-(acetylnitrosoamino)-, benzyne from 93
Benzoic acid, Birch reduction 103—104
Benzoin see “Ethanone 2-hydroxy-1
Benzophenone = diphenylmethanone, aldol addn. and condensation with 56—57
Benzoquinones see “Cyclohexadienediones”
Benzvalene = ![$tricyclo[2.1.1.0^{5,6}]hex-2-ene$](/math_tex/c12ff88955e52ef716bca43fc8b9592682.gif) 330—331 330—331
Benzyl protecting group see “Protection”
Benzyltrimethylammonium see “Benzenemethanaminium”
Benzyne see “1
Berberine alkaloids, synthesis steps 295—297
Berberine, pr. 290
Betaines in Wittig olerinations 29—30
Bi-1-cyclohex-1-enyl, synthesis steps 53 140
Bi-2, 4-cyclopentadien-1-yl 335—336
Bicyclic compounds see “Oligocyclic compounds”
Bicyclic hydrocarbon derivs., pr. 192
Bicyclo[2.2.1]heptane derivs., pr. 192
Bicyclo[2.2.1]heptane derivs., synth. by [2+2]cycloaddn. and rearr. 276
Bicyclo[2.2.1]heptane derivs., synth. by [2+4]cycloaddn 85 92 209—210
Bicyclo[2.2.1]heptane-2-ones, Baeyer — Villiger oxn. 136 210 276
Bicyclo[2.2.1]heptane-2-ones, redn. 109 141
Bicyclo[2.2.2]octane derivs. 85 92—93 331
Bicyclo[3.3.1]non-2-en-9-ones 93
Bicyclo[4.1.0]hept-2-ene, 3,7,7-trimethyl-, (+)-(1S)- ((+)-(1S)-2-carene), allylbis(2-isocaranyl)boranes from 67—68
Bicyclo[4.1.0]hept-2-ene, 3,7,7-trimethyl-, (+)-(1S)- ((+)-(1S)-2-carene), pr. 192
Bicyclo[4.1.0]hept-3-ene, 3,7,7-trimethyl-, (+)-(1S)- ((+)-(1S)-3-carene), allylbis(4-isocaranyl)boranes from 67—68
Bicyclo[4.1.0]hept-3-ene, 3,7,7-trimethyl-, (+)-(1S)- ((+)-(1S)-3-carene), pr. 192
Bicyclo[4.1.0]hepta-2, 4-dienes, rearr. 334
Bicyclo[4.1.0]heptane-2,5-diones, redn. 76
Bicyclo[4.2.0]octa-1,3,5-triene derivs., Diels — Alder addn. with 280—281 297
Bicyclo[4.2.0]octa-1,3,5-triene derivs., synthesis 80 280—281
Bicyclo[4.2.0]octa-1,3,5-triene-7,8-dione, benzene synthesis from 78
Bicyclo[4.2.0]octa-1,3,5-triene-7,8-dione, Wittig olefination 32—33
Bilayer lipid membrane (BLM) 350—351
Bilines, 10,23-dihydro-21H-, porphyrins from 349
Biochemical methods see “Enzymatic reactions” “Nucleotides” “Peptides”
Biomembrane models 350—351
Biotechnological procedures in steroid synthesis 278
Biotechnological procedures, genetic engineering 225—227 350—351
Biotechnological procedures, literature 118
Birch reduction 97—98
Birch reduction of  -acyloxy ketones 156 -acyloxy ketones 156
Birch reduction of  -oxo carboxylic ester enol ethers 114—115 -oxo carboxylic ester enol ethers 114—115
Birch reduction of (activated) alkenes 73 103—104 278
Birch reduction of alkynes 100—101
Birch reduction of arenes 87 103—104 278
Birch reduction of haloalkenes 337
Birch reduction of nitriles (reductive cleavage) 280
Bischler — Napieralski cyclization 293
BLM = bilayer lipid membrane 350—351
Blocking in solid-phase biopolymer synth. 215—224 229 232—236
Blocking of functional groups see “Protection”
Boc protecting group 162—163 (see also “Peptides”)
BOC-ON, reagent for Boc-protection 327
Bola amphiphiles, monolayer membranes 351
Borane ( ), ), ![$(2-propenyl)bis([(1R)-1\alpha,2\alpha,3\alpha,6\alpha]-3,7,7-trimethylbicyclo[4.1.0]hept-3-yl)$](/math_tex/a7d720bbd31fe9fe0b9cae9eae29f5ad82.gif) -, enantioselective allylation with 67—68 -, enantioselective allylation with 67—68
Borane ( ), ), ![$(2-propenyl)bis([(1S)-1\alpha,2\alpha,3\alpha,6\alpha]-3,7,7-trimethylbicyclo[4.1.0]hept-2-yl)$](/math_tex/5b05214317443f4d633f26288be3377682.gif) -, enantioselective allylation with 67—68 -, enantioselective allylation with 67—68
Borane ( ), (1,1,2-trimethylpropyl)- (thexylborane), hydration of dienes 130—131 ), (1,1,2-trimethylpropyl)- (thexylborane), hydration of dienes 130—131
Borane ( ), (1,1,2-trimethylpropyl)- (thexylborane), reductive coupling of alkenes 37—38 ), (1,1,2-trimethylpropyl)- (thexylborane), reductive coupling of alkenes 37—38
Borane ( ), (1,1,2-trimethylpropyl)- (thexylborane), synthesis of ketones from CO 47—48 ), (1,1,2-trimethylpropyl)- (thexylborane), synthesis of ketones from CO 47—48
Borane ( ), bis((1S,2endo,3exo)-2,6,6-trimethylbicyclo-[3.1.1]hept-3-yl)- (diisopinocampheylborane), asym. hydration of alkenes 108 ), bis((1S,2endo,3exo)-2,6,6-trimethylbicyclo-[3.1.1]hept-3-yl)- (diisopinocampheylborane), asym. hydration of alkenes 108
Borane ( ), bis(1,2-dimethylpropyl)- (diisamylborane), hydration of alkynes 131 ), bis(1,2-dimethylpropyl)- (diisamylborane), hydration of alkynes 131
Borane ( ), chlorobis((1R,2endo,3exo)-2,6,6-trimethyl-bicyclo[3.1.1]hept-3-yl)- ( ), chlorobis((1R,2endo,3exo)-2,6,6-trimethyl-bicyclo[3.1.1]hept-3-yl)- ( ), enantioselective reduction of ketones 108 ), enantioselective reduction of ketones 108
Borane ( ), hydration of alkenes 107 117 130 319—320 325 327 ), hydration of alkenes 107 117 130 319—320 325 327
Borane ( ), hydroboration of alkenes 37 89—90 96 ), hydroboration of alkenes 37 89—90 96
Borane ( ), hydroboration of alkynes 37—38 ), hydroboration of alkynes 37—38
Borane ( ), reduction with 97—98 ), reduction with 97—98
Borane ( ), reduction with, of carboxamides 111 247 ), reduction with, of carboxamides 111 247
Borane ( ), tribromo-, 1,3,2-diazaborolidines from 68—69 ), tribromo-, 1,3,2-diazaborolidines from 68—69
Borane ( ), tribromo-, cleavage of methyl ethers by 157 ), tribromo-, cleavage of methyl ethers by 157
Borane ( ), trichloro-, cleavage of methyl ethers by 157 ), trichloro-, cleavage of methyl ethers by 157
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, 1,3-dithianation of aldehydes 328 ), Lewis acid catalyst, 1,3-dithianation of aldehydes 328
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, aldol type reactions 58—59 93 ), Lewis acid catalyst, aldol type reactions 58—59 93
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, alkynylation of oxiranes 64—65 325 328 ), Lewis acid catalyst, alkynylation of oxiranes 64—65 325 328
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, allyl-stannylation of carbonyl groups 66—67 ), Lewis acid catalyst, allyl-stannylation of carbonyl groups 66—67
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, cleavage of methyl ethers 157 275 ), Lewis acid catalyst, cleavage of methyl ethers 157 275
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, cyclization of terpenes 91 ), Lewis acid catalyst, cyclization of terpenes 91
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, cyclodimerization of alkynes 329—330 ), Lewis acid catalyst, cyclodimerization of alkynes 329—330
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, reduction of esters to ethers 110 ), Lewis acid catalyst, reduction of esters to ethers 110
Borane ( ), triiluoro- ( ), triiluoro- ( ), Lewis acid catalyst, tert-butyl etherification with isobutene 229 ), Lewis acid catalyst, tert-butyl etherification with isobutene 229
Boranes, diorganyl(2-propenyl)-, chiral, enantioselective allylation of aldehydes 67—69
Boranes, triorganyl-, carbanion transfer 9 21
Boranes, triorganyl-, coupling of organyls 37—38
Boranes, triorganyl-, oxidation with N-oxides 37
| Boranes, triorganyl-, oxidative cleavage 47—48 130—132
Boranes, triorganyl-, reaction with  -unsatd. carbonyl compds. 21—22 -unsatd. carbonyl compds. 21—22
Boranes, triorganyl-, reaction with carbenoid reagents 9
Boranes, triorganyl-, reaction with carbon monoxide 9 47—48
Boranes, triorganyl-, reaction with ylides 9
Borate(1-), cyanotrihydro-, N,N,N-tributyl-1-butanaminium, redn. of aldehydes 105—106
Borate(1-), cyanotrihydro-, sodium, reduction with, of imines and oximes 112
Borate(1-), cyanotrihydro-, sodium, reduction with, of tosylhydrazones 109
Borate(1-), hydrotris(1-methylpropyl)-, lithium ( ) 105 107 322—323 327—328 ) 105 107 322—323 327—328
Borate(1-), tetrahydro-, sodium, +  , reduction of esters to ethers 110 , reduction of esters to ethers 110
Borate(1-), tetrahydro-, sodium, +  , 1-reduction of 2-enones 107 , 1-reduction of 2-enones 107
Borate(1-), tetrahydro-, sodium, +  or acids, reduction of carboxamides 111—112 or acids, reduction of carboxamides 111—112
Borate(1-), tetrahydro-, sodium, +  , 2,3-reduction of 2-enones 322—323 , 2,3-reduction of 2-enones 322—323
Borate(1-), tetrahydro-, sodium, reduction with 96—98
Borate(1-), tetrahydro-, sodium, reduction with, of aldehydes 96 98 265
Borate(1-), tetrahydro-, sodium, reduction with, of carboximidic esters 13 22—23 63—64 111—112 298
Borate(1-), tetrahydro-, sodium, reduction with, of imines 112—113
Borate(1-), tetrahydro-, sodium, reduction with, of ketones 96 98 274—275 283
Borate(1-), tetrahydro-, sodium, reduction with, of pyridinium ions 112—113
Borate(1-), tetrahydro-, zinc, reduction with, of  -unsaturated ketones 273 -unsaturated ketones 273
Borate(1-), tetrahydro-, zinc, reduction with, of ketones 319—320
Borate(1-), tris(1,2-dimethylpropyl)hydro-, lithium ( ) 107 ) 107
Borate(1-), tris(alkylthio)hydro-, sodium, reduction of aldehydes 105—106
Borinic acid, dibutyl-, enol esters 12—13
Borinic acids, diorganyl-, esters, acidolysis 38 61—62
Borinic acids, diorganyl-, esters, oxidative cleavage 60—61
Borinic acids, diorganyl-, esters, synthesis by oxn. of triorganylboranes 37—38
Borolane, 2,5-dimethyl-, (S)-trans-, chiral auxiliary group 62
Boron enolates 12—13 61—62
Boron trifluoride see “Borane trifluoro-”
Bredereck’s imidazole synthesis 151—152
Bredt’s rule see “Steric strain due to bridges”
Brefeldin, synthesis step 65
Breslow’s remote oxidation 116 118 285—286
Bromoalkanes/-hydrins/-lactones see “Halo-...”
Brompheniramine,  - 303 - 303
Buckminsterfullerenes (BF) = Fullerenes 357
Bullvalene = ![$tricyclo[3.3.2.0^{2,8}]deca-3,6,9-triene$](/math_tex/ac0dcf438b39e9d750c2dd26df92595a82.gif) 332—333 332—333
Butanal, 2-(hydroxymethyl)-2-methyl- 203
Butanal, 3-methyl-, retro-synthesis 197—199
Butane, 1,4-dibromo-, spiroaneliation with 24 300
Butane, 2-bromo-, (-)-(R)-, stereoselective alkylation with 36
Butanedioic acid, 2,3-dihydroxy- (tartaric acid), derivs., chiral esters (DET, DIPT, DMT), epoxidation catalysts from 124—127 265 321—325
Butanedioic acid, 2,3-dihydroxy- (tartaric acid), derivs., opt. resolution with 300 301 309 327
Butanedioic acid, 2,3-dihydroxy- (tartaric acid), derivs., oxidative cleavage 202
Butanedioic acid, 2,3-dihydroxy- (tartaric acid), derivs., pr. 185
Butanedioic acid, 2-hydroxy- (malic acid), esters, Frater — Seebach 3-alkylation 27
Butanedioic acid, 2-hydroxy- (malic acid), pr. 183
Butanedioic acid, 2-[[2,6-bis(1-methylethoxy)-benzoyl]oxy]-3-hydroxy-, (R,R)- and (S,S)-, chiral aldol addition catalyst from 61
Butanedioic acid, esters, 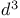 -synthon 14 -synthon 14
Butanedioic acid, esters, cyclopentanellation with 58—59
Butanedioic acid, various derivatives, 1,3-cyclopentanediones from 81
Butanoic acid, 3-hydroxy-, esters, Frater — Seebach 2-alkylation 27
Butanoic acid, 3-hydroxy-, esters, pr. 176
Butanoic acid, 3-oxo-, esters (acetoacetic esters), 2-oxopropyl 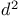 -synthon 205 -synthon 205
Butanoic acid, 3-oxo-, esters (acetoacetic esters), carbon acidity 10
Butanoic acid, 3-oxo-, esters (acetoacetic esters), carboxymethyl 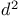 -synthon 19 207 -synthon 19 207
Butanoic acid, 3-oxo-, esters (acetoacetic esters), dianion, 4-alkylation 24 207 325—326
Butanoic acid, 3-oxo-, esters (acetoacetic esters), pr. 176
Butyric acid see “Butanoic acid”
Cadiot — Chodkiewicz coupling 40
Caffeine, pr. 290
Caffeine, synthesis 306
Calciferols (vitamin D) 31—32 281—283 289
Calcium(2+), effect on biomembranes 243—244
Camphor, pr. 192
Camphor, reduction of hydrazones 109 141
Can see “Cerate(2-) hexanitrato- diammonium”
Cantharidin =  -hexahydro-3a,7a-dimethyl-4,7-epoxyisobenzofuran-1,3-dione, synthesis steps 81—82 86 -hexahydro-3a,7a-dimethyl-4,7-epoxyisobenzofuran-1,3-dione, synthesis steps 81—82 86
Caramiphen 300
Carbanions 4—5 (see also “Organometallic compounds”)
Carbanions, silylated 6—7 28 33—34
Carbanions, stabilized see “Donor synthons”
Carbenes = methylenes 74—75 94 141—142
Carbenes = methylenes, chlorocarbene 330
Carbenes = methylenes, dibromocarbene 75
Carbenes = methylenes, ethoxycarbonylcarbene 75
Carbenes = methylenes, methoxycarbene 74
Carbenoid synthons, cyclopropanes from 74—76 330 334
Carbenoid synthons, insertions with 75 83 141 166
Carbocations (carbenium ions) see “Friedel — Crafts acylation” “Friedel
Carbocations (carbenium ions), acylium and alkylium ions 15—16
Carbocycles, 10-membered, synthesis from fused ring systems 89—90 332
Carbocycles, 12-membered, pr. 191
Carbocycles, 12-membered, synthesis 41
Carbocycles, 15-membered, synthesis 41
Carbocycles, 16-membered, pr. 191
Carbocycles, 3-membered, pr. 189
Carbocycles, 3-membered, ring expansion of 76—78 79 83 298—299 335—336
Carbocycles, 3-membered, ring opening of 69—70 76—77 105 276 288—289
Carbocycles, 3-membered, synthesis of, by cyclopropanation, with carbenoids 74—76 94 330 334
Carbocycles, 3-membered, synthesis of, by cyclopropanation, with sulfur ylides 75—76
Carbocycles, 3-membered, synthesis of, by extrusion of 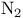 330—331 334 330—331 334
Carbocycles, 3-membered, synthesis of, by homoallylic 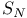 reaction 288—289 reaction 288—289
Carbocycles, 3-membered, synthesis of, by intramol. 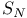 reaction 276 298 reaction 276 298
Carbocycles, 3-membered, synthesis of, by intramol. carbene insertion 75 92
Carbocycles, 3-membered, synthesis of, by oxidative ring contraction 129—130 136
Carbocycles, 4-membered, pr. 189
Carbocycles, 4-membered, ring contraction of 130 136
Carbocycles, 4-membered, ring expansion of 79 83
Carbocycles, 4-membered, ring opening of 53—54 79—80 280—281 297
Carbocycles, 4-membered, synthetic methods 77—80
Carbocycles, 4-membered, synthetic methods by pyrolytic extrusions 80
Carbocycles, 4-membered, synthetic methods by ring contraction 78
Carbocycles, 4-membered, synthetic methods by ring expansion 77 79—80 335—336
Carbocycles, 4-membered, synthetic methods, acyloin coupling 53—54
Carbocycles, 4-membered, synthetic methods, electrocyclizations 330 333
Carbocycles, 4-membered, synthetic methods, intramol. alkylation 3 23 280
Carbocycles, 4-membered, synthetic methods, intramol. alkyne cyclotrimerization 80 281
Carbocycles, 4-membered, synthetic methods, photolytic extrusion of CO or Na 329—330
Carbocycles, 4-membered, synthetic methods, pinacol coupling 53
Carbocycles, 4-membered, synthetic methods, [2+2]cycloaddition 78 83 276 297—298 329—330 332—333 336—337
Carbocycles, 4-membered, synthetic methods, [2+2]cyclodimerization of alkynes 329—330
Carbocycles, 4-membered, [2+2]cycloreversion of 79—80 332 333
Carbocycles, 5-membered, pr. 189
Carbocycles, 5-membered, ring expansion via ozonolysis 91—92 280
Carbocycles, 5-membered, synthetic methods 81—85
Carbocycles, 5-membered, synthetic methods, acid-catalyzed 1-en-5-yne cyclizations 91—92 279—280
Carbocycles, 5-membered, synthetic methods, acyloin coupling 53—54
Carbocycles, 5-membered, synthetic methods, aldol type reactions 69 79 81—83
Carbocycles, 5-membered, synthetic methods, carbonylation of cyclic boranes 48
Carbocycles, 5-membered, synthetic methods, ester condensations 3 55 59 82—83
Carbocycles, 5-membered, synthetic methods, Horner olefination 29
Carbocycles, 5-membered, synthetic methods, intramol. alkylation 24 90 93 300
Carbocycles, 5-membered, synthetic methods, intramol. Diels — Alder addition 84
Carbocycles, 5-membered, synthetic methods, McMurry coupling 41
Carbocycles, 5-membered, synthetic methods, metal-catalysed 1,6-enyne cyclization 84
Carbocycles, 5-membered, synthetic methods, pinacol coupling 53
Carbocycles, 5-membered, synthetic methods, rearr. of (1-alkenyl)cyclopropanes 77 83
Carbocycles, 5-membered, synthetic methods, ring contraction 81—82 84 130 211 337
Carbocycles, 5-membered, synthetic methods, ring expansion 79 83
Carbocycles, 5-membered, synthetic methods, ring opening of bicyclo[2.2.1]heptanes 210 276
Carbocycles, 5-membered, synthetic methods, samarium(2+) coupling 69
Carbocycles, 5-membered, synthetic methods, special cyclopentanellations 59 81 83 84
Carbocycles, 5-membered, synthetic methods, spiroannellation with 1,4-dihalides, 1,4-ditosylates 23—24 300
Carbocycles, 5-membered, synthetic methods, [2+3]cycloaddition 84
Carbocycles, 6-membered, pr. 189—191
Carbocycles, 6-membered, ring contraction of 78 81—82 84 130 211 337
Carbocycles, 6-membered, ring expansion of, via fused ring systems 76 89—90 332 334
Carbocycles, 6-membered, ring opening of 81—82 87—90 136—137 206
Carbocycles, 6-membered, special syntheses, 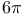 -electrocyclization 38 295—296 338 -electrocyclization 38 295—296 338
Carbocycles, 6-membered, special syntheses, acid-catalyzed 1,5-diene cyclizations 90—92 279—280
Carbocycles, 6-membered, special syntheses, benzo-anellation procedure 337
Carbocycles, 6-membered, special syntheses, reduction of arenes 87 104 208 278
Carbocycles, 6-membered, special syntheses, ring expansion of cyclopentenes 91—92 280
Carbocycles, 6-membered, special syntheses, Robinson anellation 71—73 298
Carbocycles, 6-membered, special syntheses, spiroanellation with bis-Michael type reagents 74
Carbocycles, 6-membered, special syntheses, tetracycline synthesis 318
Carbocycles, 6-membered, special syntheses, [2+2+2]cyclotrimerization 80 281 330
Carbocycles, 6-membered, special syntheses, [2+4]cycloaddn 80 81—82 85—86 92—93 153 280—281 297 331 334—337
|
|
 |
| Ðåêëàìà |
 |
|
|
 |
|
Î ïðîåêòå
|
|
Î ïðîåêòå



 )
)  )
) ![$pentacyclo[4.4.0.0^{2,5}.03^{3,8}.0^{4,7}]dec-9-ene$](/math_tex/579817118bbad24592ab7a04af75574882.gif) , photo dimer
, photo dimer  -hydroxy-aldehydes
-hydroxy-aldehydes  ), prepn. of
), prepn. of ![$\mathrm{Me_{2}Zn[TiCl_{4}]}$](/math_tex/0f70b1c11e62aee366c0b1ebc400f2b582.gif) , Reetz carbosulfenylation of alkenes
, Reetz carbosulfenylation of alkenes  ), bromohydrination with
), bromohydrination with ![$tricyclo[2.1.1.0^{5,6}]hex-2-ene$](/math_tex/c12ff88955e52ef716bca43fc8b9592682.gif)
 -oxo carboxylic ester enol ethers
-oxo carboxylic ester enol ethers  ),
), ![$(2-propenyl)bis([(1R)-1\alpha,2\alpha,3\alpha,6\alpha]-3,7,7-trimethylbicyclo[4.1.0]hept-3-yl)$](/math_tex/a7d720bbd31fe9fe0b9cae9eae29f5ad82.gif) -, enantioselective allylation with
-, enantioselective allylation with ![$(2-propenyl)bis([(1S)-1\alpha,2\alpha,3\alpha,6\alpha]-3,7,7-trimethylbicyclo[4.1.0]hept-2-yl)$](/math_tex/5b05214317443f4d633f26288be3377682.gif) -, enantioselective allylation with
-, enantioselective allylation with  ), enantioselective reduction of ketones
), enantioselective reduction of ketones  ), Lewis acid catalyst, 1,3-dithianation of aldehydes
), Lewis acid catalyst, 1,3-dithianation of aldehydes  -unsatd. carbonyl compds.
-unsatd. carbonyl compds.  )
)  , 1-reduction of 2-enones
, 1-reduction of 2-enones  or acids, reduction of carboxamides
or acids, reduction of carboxamides  , 2,3-reduction of 2-enones
, 2,3-reduction of 2-enones  )
)  -
- ![$tricyclo[3.3.2.0^{2,8}]deca-3,6,9-triene$](/math_tex/ac0dcf438b39e9d750c2dd26df92595a82.gif)
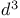 -synthon
-synthon 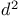 -synthon
-synthon  -hexahydro-3a,7a-dimethyl-4,7-epoxyisobenzofuran-1,3-dione, synthesis steps
-hexahydro-3a,7a-dimethyl-4,7-epoxyisobenzofuran-1,3-dione, synthesis steps 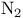
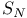 reaction
reaction 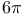 -electrocyclization
-electrocyclization