|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. Ч Organic Synthesis: Concepts, Methods, Starting materials |

ќбсудите книгу на
Ќашли опечатку?
¬ыделите ее мышкой и нажмите Ctrl+Enter
|
Ќазвание: Organic Synthesis: Concepts, Methods, Starting materials
јвторы: Fuhrhop J., Penzlin G.
язык: 
–убрика: ’ими€/
—татус предметного указател€: √отов указатель с номерами страниц
ed2k:
»здание: second edition, rewise and enlarged
√од издани€: 1994
оличество страниц: 432
ƒобавлена в каталог: 29.10.2005
ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
|
|
 |
| ѕредметный указатель |
Ferrate(2-), tetracarbonyl-, formyl d-synthon 18 46Ч47 198
Ferrate(2-), tetracarbonyl-, redn. of acid anhydrides and halides with 46Ч47
Ferrate(3-), hexakis(cyano-C)-, potassium ( ), ), 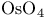 -catalysed oxn. with 129 -catalysed oxn. with 129
FGA, retro-synthetic 195
FGI 95
FGI, retro-synthetic 195
FGR, retro-synthetic 195
Fischer formula, erythro/threo definition 62 360
FischerТs indole synthesis 151Ч152 296 307
FischerТs porphyrin synthesis 256
FK 506, total synthesis 324Ч329
Fluoride, hydrogen, cleavage with, of diol acetonides 277
Fluoride, hydrogen, cleavage with, of silyl ethers 277 328 329
Fluoride, hydrogen, cleavage with, of silyl thioethers 169
Fluoride, potassium, base cat. for Michael addn. 71
Fluoride, silver, halide/fluoride exchange 269Ч270
Fluoride, tetrabutylammonium, cleavage of silyl ethers 157 159 270 272 281 339
Fluorination with 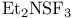 (DAST), OH/F exchange 269Ч270 272 (DAST), OH/F exchange 269Ч270 272
Fluorination with 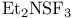 (DAST), SR/F exchange ( (DAST), SR/F exchange (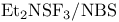 ) 269Ч270 ) 269Ч270
Fluorination with  , oxidation of alkanes 118Ч119 157 , oxidation of alkanes 118Ч119 157
Fluorosulfuric acid, methyl ester 338
Formaldehyde, hydroxymethyl 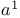 -synthon 18 198 203 302 -synthon 18 198 203 302
Formaldehyde, Mannich reactions 57 292 300 304 310 317Ч318
Formaldehyde, reduction of aldehydes with 302
Formaldehyde-sulfoxylate see УMethanesulfinic acid hydroxy-Ф
Formamides, N,N-diaikyl-, formyl 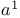 -synthons 16 18 198 255 -synthons 16 18 198 255
Formic acid, azido- see УCarbonazidic acidФ
Formic acid, chloro- see УCarbonochloridic acidФ
Formic acid, esters, formyl 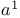 -synthons 18 58Ч59 155Ч156 337 -synthons 18 58Ч59 155Ч156 337
Formic acid, esters, orthoesters see УOrthoformic acid estersФ
Formylation, 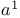 -synthons 16 18 -synthons 16 18
Formylation, 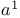 -synthons, formic esters 59 155Ч156 -synthons, formic esters 59 155Ч156
Formylation, 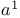 -synthons, orthoformamidic esters 79 82 -synthons, orthoformamidic esters 79 82
Formylation, 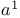 -synthons, orthoformic esters/trifluoroborane 59 -synthons, orthoformic esters/trifluoroborane 59
Formylation,  -synthons 6Ч8 18 -synthons 6Ч8 18
Formylation,  -synthons, (dichloromethyl)lithium 51Ч52 -synthons, (dichloromethyl)lithium 51Ч52
Formylation,  -synthons, 1,3-dithianes 17Ч18 198 267 -synthons, 1,3-dithianes 17Ч18 198 267
Formylation,  -synthons, allyl or vinyl d-synthons + -synthons, allyl or vinyl d-synthons +  90 90
Formylation,  -synthons, boranes + carbon monoxide 47Ч48 -synthons, boranes + carbon monoxide 47Ч48
Formylation,  -synthons, cyanide anion 50 -synthons, cyanide anion 50
Formylation,  -synthons, DMSO anion 51 -synthons, DMSO anion 51
Formylation,  -synthons, isocyanomethyl sulfones (TosMIC) 49 51 -synthons, isocyanomethyl sulfones (TosMIC) 49 51
Formylation,  -synthons, nitromethane anion 65Ч66 -synthons, nitromethane anion 65Ч66
Formylation,  -synthons, tetracarbonylferrate(2-) 46Ч47 -synthons, tetracarbonylferrate(2-) 46Ч47
Formylation, reductive, (alkoxymethylene)phosphoranes 6 49
Fragmentation reactions 89Ч90 141Ч142
Frater Ч Seebach alkylation 27 325Ч326
Friedel Ч Crafts acylation, 1-oxoalkylium (acylium) ions in 15Ч16
Friedel Ч Crafts acylation, aldol type cyclizations by 81
Friedel Ч Crafts alkylation of dipyrromethenes 256Ч257
Friedel Ч Crafts alkylation of indoles 291
Friedel Ч Crafts alkylation of polystyrene 233
Friedel Ч Crafts alkylation, alkylium ions in 15Ч16
Fullerenes 357
Fullerenes, metal inclusion compounds 357
Fulvalene see УBi-2
Functional group addition (FGA), rerro-synthetic 195
Functional group interconversion (FGI) 95Ч169
Functional group interconversion (FGI), retro-synthetic 195
Functional group removal (FGR), retro-synthetic 195
Functional group, definition 1Ч4 50
Furan, Diels Ч Alder addition to 86 93
Furosemide 305
Gene cloning 241Ч246
Genetic engineering methods 225Ч227 241Ч246
Geraniol see У2 3
Geranyl bromide see У2 1-bromo-3
Gipsy moth pheromone 34 125
Girgnard tranform, retro-synthetic 194
Glaser coupling 40
Glass, porous, solid support 221 342
Glucal derivatives 268
Glucosamine, pr. 263
Glucosamine, synthesis 268
Glucose derivatives, pr. 263Ч264
Glucose derivatives, syntheses of 266Ч269
Glucose derivatives, syntheses starting from 267Ч273
Glutethimide,  - 304 - 304
Glycals 268
Glycine, N-benzoyl,  synthon 48 synthon 48
Glycols see УDiolsФ
Glycosyl fluorides 269Ч270 272
Glycosyl halides,  -elimination, 2-acetoxyglycals 268 -elimination, 2-acetoxyglycals 268
Glycosyl halides, glycosylations with 267 269Ч271
Glycosyl halides, prepn. 266 269Ч270 272
Glycosyl halides, reductive  -elimination, glycals 268 -elimination, glycals 268
Glycosyl imidates 270Ч271
Glycosylations 267 269Ч272
Glyoxylic acid = oxoacetic acid, derivs., deblocking of acetals with 328Ч329
Glyoxylic acid = oxoacetic acid, derivs., pr. 176
Glyoxylic acid = oxoacetic acid, derivs., synthesis 202
Grignard reagents see УMagnesium alkylhalo-Ф
Griseofulvin,  -, synthesis 74 -, synthesis 74
Griseofulvin, dehydro-,  -, synthesis 86 -, synthesis 86
Guanidine group, protection in arginine 229
Hafnium,  -, + -, + 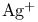 , activation of glycosyl fluorides 270 , activation of glycosyl fluorides 270
Haloalkanes, alkenes from see У -EliminationФ -EliminationФ
Haloalkanes, alkyl a-synthons 15Ч16 22Ч27
Haloalkanes, conversion to phosphines 282
Haloalkanes, conversion to thiols 169
Haloalkanes, coupling of 19Ч20 36
Haloalkanes, metalation (= umpolung) 5Ч7 17 20 70 75 326Ч327
Haloalkanes, reduction of 97 113Ч115 203
Haloalkanes, reduction of, with alkali metals/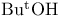 (Birch redn.) 337 (Birch redn.) 337
Haloalkanes, reduction of, with chromium(2 +) acetate 286
Haloalkanes, reduction of, with hydrogen/Raney Ni 287
Haloalkanes, reduction of, with methyllithium/lithium bromide 75
Haloalkanes, reduction of, with tributylstannane 97 114Ч115 275 319Ч320
Haloalkanes, reduction of, with zinc or zinc-copper 83 335Ч336
Haloalkanes, synthesis from alcohols, bromination, conc. aq.  303 303
Haloalkanes, synthesis from alcohols, chlorination, N-Chlorosuccimmide/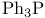 169 169
Haloalkanes, synthesis from alcohols, chlorination, N-Chlorosuccimmide/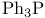 , fluorination with , fluorination with 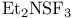 269Ч270 272 269Ч270 272
Haloalkanes, synthesis from alcohols, chlorination, N-Chlorosuccimmide/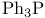 , glycosyl halides 266 269Ч270 272 , glycosyl halides 266 269Ч270 272
Haloalkanes, synthesis from alcohols, chlorination, N-Chlorosuccimmide/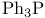 , iodides via tosylates 203 281 , iodides via tosylates 203 281
Haloalkanes, synthesis from alcohols, chlorination, N-Chlorosuccimmide/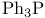 , iodides, iodine/ , iodides, iodine/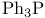 328 328
Haloalkanes, synthesis from alcohols, chlorination, N-Chlorosuccimmide/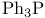 , Mitsunobu reactions with , Mitsunobu reactions with  160Ч161 160Ч161
Haloalkanes, synthesis from alcohols, chlorination, N-Chlorosuccimmide/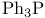 , tetrahalomethane/ , tetrahalomethane/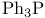 266 355 266 355
Haloalkanes, synthesis from alcohols, chlorination, N-Chlorosuccimmide/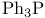 , thionyl chloride ( , thionyl chloride (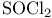 ) 32 ) 32
Haloalkanes, synthesis from alcohols, chlorination, N-chlorosuccinimide 282
Haloalkanes, synthesis from alcohols, chlorination, synthesis from thioethers 269Ч270
Halohydrins (vic-halo alcohols), synthesis of, from 1,2-diols 160 327Ч328
Halohydrins (vic-halo alcohols), synthesis of, from alkenes 77 117 275Ч276 287
Halohydrins (vic-halo alcohols), synthesis of, from oxiranes, with HF 287
Halolactonization 275 319Ч321 335Ч336
HauserТs rule 9 24 204
HBT = 1-hydroxy-1H-benzotriazole 145 231
Heck coupling (alkene + organyl halide) 42Ч43
Hectane, synthesis 36
Helicate complexes 345Ч346
Hemiacetals, cyclic, from lactones ( ) 105 321 322 327Ч328 ) 105 321 322 327Ч328
Heterocycles see УAlkaloidsФ УDrugsФ УN-HeterocyclesФ
Heterocycles, 3-membered see УAziridinesФ УOxiranesФ УThiiranesФ
Heterocycles, macrocyclic see УMacrocyclesФ
Hexamethyldisilazane see УStlanamine ...Ф
Hexanoic acid, 2-methyl-, (S)-, synth. 22Ч23
Hexonamides, N-alkyl-, aqueous suspensions, various micellar aggregates 352Ч353
High-dilution methods, macrocycle syntheses by 145Ч146 240Ч241 329 338
Hindrance, steric see УSteric hindranceФ
Hippuric acid see УGlycine N-benzoyl-Ф
HMDS see УSilanamine ...Ф
HMPA = HMPTA see УPhosphoric triamide hexamethyl-Ф
HOBT = 1-hydroxy-1H-benzotriazole 145 231
Hofmann degradation 139 308 331
Hofmann type elimination of ammonium compounds 57 72 138 140Ч141 331
Hofmann type elimination of sulfonium compounds 38 338
Hofmann type elimination of sulfoxides 65 86 315
Homo-DNA 344Ч345
homo-Michael-type additions,  69Ч70 69Ч70
homo-Michael-type additions, 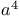 + alkenyl d 276 + alkenyl d 276
Homoenolate synthons 14Ч15 70 76
| Horner olefination 28Ч30 (see also УPhosphonic acids alkyl- diestersФ)
Huenig base see У1-Naphthalenamine N
Huenig bases, polymeric = aminated chloromethylated ethenylbenzene homopolymers, deprotonation with, of ketones 11
Huenig bases, polymeric = aminated chloromethylated ethenylbenzene homopolymers, deprotonation with, or phosphonium salts 32
Huenig bases, polymeric = aminated chloromethylated ethenylbenzene homopolymers, removal of acids with 32
Hybrid plasmids 243Ч245
hydration see УAlkenesФ УAlkynesФ
Hydrazine, hydrazinolysis with, of esters 239Ч240 331
Hydrazine, hydrazinolysis with, of phthaloyl-protected amines 162 163
Hydrazine, reduction of ketones with 97Ч98 109
Hydrazines, cyclic, oxn. 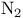 extrusion 35 331 extrusion 35 331
Hydrazones, aryl-, indole synth. with 151Ч152 296 307
Hydrazones, dialkyl-, lithiated, alkylation 12 18 25Ч26
Hydrazones, dialkyl-, lithiated, alkylation, enantioselective (SAMP-hydrazones) 25Ч26
Hydrazones, dialkyl-, lithiated, ozonolysis of 26
Hydrazones, Wolff Ч Kishner redn. of 109
Hydrazones, [(4-methylphenyi)sulfonyl]- (tosylhydrazones), fragmentation of 89 141Ч142
Hydrazones, [(4-methylphenyi)sulfonyl]- (tosylhydrazones), reduction of, to alkanes 109
Hydrazones, [(4-methylphenyi)sulfonyl]- (tosylhydrazones), reduction of, to alkenes 141Ч142
Hydrides of Al, B, Sn see УAluminumФ УAluminateФ УBoraneФ УBorateФ УStannaneФ
Hydrides, alkali, deprotonation with, of alcohols 23 33 157 325 328
Hydrides, alkali, deprotonation with, of carbonyl compounds 15 49 58 63 93
Hydrides, alkali, deprotonation with, of carboxamides 314
Hydrides, alkali, deprotonation with, of indoles 307
Hydrides, alkali, deprotonation with, of phosphonic diesters 29 334
Hydrides, alkali, deprotonation with, of sulfonium salts 38 45
Hydrides, alkali, deprotonation with, of sulfoxides 6 51
Hydrides, alkali, deprotonation with, of tosylhydrazones 35
Hydroalkylation of alkenes 37 105
Hydroalkylation of alkynes 37Ч38
Hydroboration see УAlkenesФ УAlkynesФ
Hydrobromic acid (aq. HBr), OH/Br exchange (aq.  ) 303 ) 303
Hydrobromic acid (aq. HBr), porphyrin synthesis with 255 349
Hydrobromination of alkynes with Zr 132 325
Hydrochlorothiazide 310
Hydrofluoric acid (aq. HF), deblocking with, of diol acetonides 277
Hydrofluoric acid (aq. HF), deblocking with, of silyl ethers 277 329
Hydrofluoric acid (aq. HF), deblocking with, of silyl thioethers 169
Hydroformylation of alkenes 46Ч48
Hydrogen peroxide, epoxidation with, of 2-enones (with KOH) 274Ч275
Hydrogen peroxide, epoxidation with, of alkenes (with HCOOH) 123
Hydrogen peroxide, oxidation with, of phenylselenyl groups 139
Hydrogen peroxide, oxidation with, of phosphines to P-oxides 282
Hydrogen peroxide, oxidative cleavage of 2-enones 82
Hydrogen peroxide, oxidative cleavage of phosphorus ylides 31
Hydrogen peroxide, oxidative cleavage or triorganylboranes 47Ч48 130Ч131 325
Hydrogen peroxide, oxidative rearr. (Baeyer Ч Villiger) of carbonyl compounds with  136Ч137 136Ч137
Hydrogen shift, sigmatropic 262 289 295 УRearrangements sigmatropicФ)
Hydrogenation 96Ч105 (see also УHydrogenolysisФ)
Hydrogenation of alkenes 41 96Ч97 101Ч103 278 283
Hydrogenation of alkynes to (Z)-alkenes 40 64 100Ч101
Hydrogenation of arenes 87 347
Hydrogenation of heteroarenes 112Ч113 303
Hydrogenation of iminium ions 293
Hydrogenation of nitroalkanes 66 112 297
Hydrogenation, catalysts, heterogeneous 96 100Ч102 105 111Ч113
Hydrogenation, catalysts, homogeneous 96Ч97 102Ч103 326
Hydrogenation, catalysts, homogeneous, chiral (Noyori) 102Ч103 325Ч326
Hydrogenation, mechanism 101Ч102
Hydrogenolysis 113Ч114 (see also УReductive cleavageФ)
Hydrogenolysis of activated keto groups 109
Hydrogenolysis of allylic alcohol derivs. 113Ч114 312Ч313
Hydrogenolysis of benzyl carbamates 143 162Ч163 229 240
Hydrogenolysis of benzyl ethers 157Ч159 161 271 328
Hydrogenolysis of benzylidene acetals 268
Hydrogenolysis of cyclopropane rings 105
Hydrogenolysis of haloalkanes 97 113Ч114 286Ч287
Hydrogenolysis of oxirane rings 113Ч114 319Ч320
Hydrogenolysis of thioethers 26 113Ч114
Hydrogenolysis of УOxФ-protected amines 161 164
hydrolysis see УDeblockingФ
Hydrolysis, enantiotoposelective 167Ч168 276Ч277
Hydroperoxide, 1,1-dimethylethyl ( , tert-butyl hydroperoxide, TBHP), Sharpless epoxidation with 124Ч127 265 321Ч325 , tert-butyl hydroperoxide, TBHP), Sharpless epoxidation with 124Ч127 265 321Ч325
Hydrostannylation of alkynes 321
Hydroxamic acid chlorides = N-hydroxy carboximidoyl chlorides 153
Hydroxides, alkali, deprotonation with, of alcohols 157 159 265
Hydroxides, alkali, deprotonation with, of dicarbonyl type compounds 10 71Ч73
Hydroxides, alkali, deprotonation with, of hydrazones 109
Hydroxides, alkali, deprotonation with, of ketones 69 78 79 82 280
Hydroxides, alkali, deprotonation with, of oxosulfonium salts 8
Hydroxides, alkali, deprotonation with, of sulfonium salts 79 336
Hydroxides, benzyltrimethylammonium see УBenzene-methanaminium ...Ф
Hydroxy acids, chiral, pr. 176 183 185 191
Hydroxylamine-HCl, cleavage of acetonides 323
Hydrozirconation of alkynes 132 325
Hyperconjugation effects of alkyl groups on Baeyer Ч Villiger rearr. 136Ч137
Hyperconjugation effects of alkyl groups on enolate formation 11Ч13 57Ч58 93
Hyperconjugation effects of alkyl groups on relative reactivities of aldehydes vs. ketones to d-synthons 56
Hyperconjugation effects of alkyl groups on relative reactivities of alkenes to oxidation 87Ч88 123Ч124 156Ч157
Hyperconjugation effects of alkyl groups on relative reactivities of prim. vs. sec. alcohols to oxidation 135
Hypochlorites,  -catd. oxidation of alkynes 132 -catd. oxidation of alkynes 132
Hypochlorites, chlorohydrination with 117
Hypochlorus acid, 1,1-dimethylethyl ester, oxidative cleavage of thioethers with 314
Hypochlorus acid, 1,1-dimethylethyl ester, oxidative rearr. of 1-amino alcohols 77Ч78
Hypoiodites, acyl-, oxn. of alkenes with 127
Imidazoles, synthesis 151Ч152 305
Imides see УDicarboximidesФ
Imidic acids see УCarboximidic acidsФ
Imido esters see УCarboximidic acids estersФ
Imidogens see УNitrenesФ
Imines, N-alkyl- = N-alkylidene amines, hydrolysis of 57
Imines, N-alkyl- = N-alkylidene amines, N-acylation of 153
Imines, N-alkyl- = N-alkylidene amines, reduction of 112Ч113
Imines, N-alkyl- = N-alkylidene amines, synthesis of, by condensation of reactive 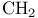 with nitroso compounds 308 with nitroso compounds 308
Imines, N-alkyl- = N-alkylidene amines, synthesis of, by trapping of diallylnickel complexes with isocyanides 41
Imines, N-alkyl-, lithiated 12 18 56Ч57 199
Imines, N-alkyl-, lithiated, directed aldol condensation with 56Ч57
Imines, synthesis of, aldehyde + amine 298
Imines, synthesis of, alkylation or reduction of nitriles 298Ч299
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), aminomethylation with, of 1-alkynes 57
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), aminomethylation with, of carboxamides 317Ч318
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), aminomethylation with, of indoles and phenols 291Ч292
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), aminomethylation with, of ketones 57 300 304
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), aminomethylation with, of pyrroles 252
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), aminomethylation with, of sulfonamides 310
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), conversion to enamines 13 88 139
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), preparation, from carbonyl compounds 13 57 292 300 304 310 318
Iminium (= N-alkyl-N-alkylidene alkanaminium = immonium) ions (Mannich reagents), preparation, oxidation of tert. amines 116 120Ч121 138Ч139
Immunosuppressive agent УFK 506Ф 324Ч329
Indoles, 2-substitution, Friedel Ч Crafts type 291
Indoles, 2-substitution, Mannich type 292
Indoles, 2-substitution, Vilsmeier type 293
Indoles, N-acylation 307
Indoles, synthesis (Fischer) 151Ч152 296 307
Indomethacin 307
INOC = [(4-pyridinylmethoxy)carbonyl)] 241
Inserted DNA sequences 243Ч245
Insulin B-chain, chemical synthesis 239Ч240
Insulin, synth. by genetic engineering 244Ч245
Introns 242
Inversion of configuration see УEpimerizationФ
Inversion of configuration of alcohols (Mitsunobu reaction) 160Ч161 286
Inversion of configuration of alkyl halides or sulfonates by 1,3-dithiane anions 22
Inversion of configuration of alkyl halides or sulfonates by organylcuprates 36
Inversion of configuration of alkyl halides or sulfonates by tetracarbonyIferrate(2-) 46Ч47
Inversion of configuration of alkyl halides or sulfonates, in triorganylborane rearr. 37Ч38
Inversion of configuration of allylic acetates with Pd 27 164
Inversion of configuration of allylpalladium with d-synthons 27 264
Inversion of configuration of glycosides with free 2-OH by DAST 272
Inversion of configuration of glycosyl halides 271
Inversion of configuration of oxiranes by 1-alkyne anions 64 204
Inversion of configuration of oxiranes by enolate type anions 63Ч64
Inversion of configuration of oxiranes by internal alcoholate addition 265
Inversion-esterification of alcohols 160Ч161 286
Iodide, hydrogen, porphyrin synthesis with 255
Iodine = diiodine (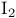 ) + ) + 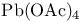 , ox. decomp. of RCOOH 337 , ox. decomp. of RCOOH 337
Iodine = diiodine (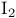 ) + carboxylic acids, oxidation of alkenes to 1,2-diols 127Ч128 ) + carboxylic acids, oxidation of alkenes to 1,2-diols 127Ч128
Iodine = diiodine (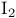 ) + hydroxide, rearr. of 1-alkenyl boranes 37 ) + hydroxide, rearr. of 1-alkenyl boranes 37
Iodine = diiodine (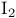 ) + triphenylphosphine, OH/I exchange 328 ) + triphenylphosphine, OH/I exchange 328
Iodine = diiodine (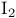 ) + water, oxn. of phosphites 219Ч220 223 ) + water, oxn. of phosphites 219Ч220 223
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 ),
), 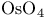 -catalysed oxn. with
-catalysed oxn. with 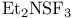 (DAST), OH/F exchange
(DAST), OH/F exchange 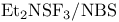 )
)  , oxidation of alkanes
, oxidation of alkanes 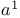 -synthon
-synthon  -synthons
-synthons 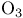
 -
-  synthon
synthon  -elimination, 2-acetoxyglycals
-elimination, 2-acetoxyglycals  -, +
-, + 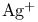 , activation of glycosyl fluorides
, activation of glycosyl fluorides 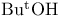 (Birch redn.)
(Birch redn.) 
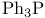

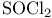 )
)  )
) 
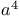 + alkenyl d
+ alkenyl d 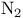 extrusion
extrusion  )
) 
 , tert-butyl hydroperoxide, TBHP), Sharpless epoxidation with
, tert-butyl hydroperoxide, TBHP), Sharpless epoxidation with  -catd. oxidation of alkynes
-catd. oxidation of alkynes 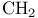 with nitroso compounds
with nitroso compounds 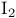 ) +
) + 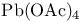 , ox. decomp. of RCOOH
, ox. decomp. of RCOOH