|
|
 |
| Àâòîðèçàöèÿ |
|
|
 |
| Ïîèñê ïî óêàçàòåëÿì |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. — Organic Synthesis: Concepts, Methods, Starting materials |

Îáñóäèòå êíèãó íà
Íàøëè îïå÷àòêó?
Âûäåëèòå åå ìûøêîé è íàæìèòå Ctrl+Enter
|
Íàçâàíèå: Organic Synthesis: Concepts, Methods, Starting materials
Àâòîðû: Fuhrhop J., Penzlin G.
ßçûê: 
Ðóáðèêà: Õèìèÿ/
Ñòàòóñ ïðåäìåòíîãî óêàçàòåëÿ: Ãîòîâ óêàçàòåëü ñ íîìåðàìè ñòðàíèö
ed2k:
Èçäàíèå: second edition, rewise and enlarged
Ãîä èçäàíèÿ: 1994
Êîëè÷åñòâî ñòðàíèö: 432
Äîáàâëåíà â êàòàëîã: 29.10.2005
Îïåðàöèè: Ïîëîæèòü íà ïîëêó |
Ñêîïèðîâàòü ññûëêó äëÿ ôîðóìà | Ñêîïèðîâàòü ID
|
|
 |
| Ïðåäìåòíûé óêàçàòåëü |
Diastereotopic ligands, definition 360
Diazene (diimide, diimine, 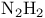 ), reduction of alkenes 97 102 ), reduction of alkenes 97 102
Diazenedicarboxylic acid, diazene from 102
Diazenedicarboxylic acid, diethyl ester, inversion-esterification with 286
Diazenedicarboxylic acid, diethyl ester, Mitsunobu reactions 160—161
Diazenes, cyclic, synthesis and 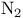 extrusion 35 331 334 extrusion 35 331 334
Diazepan 307
Diazoalkanes see “Methane diazo-”
Diazoalkanes, Barton olefination with 35
Diazoalkanes, cyclopropanation with 74—75 94
Diazotization of amino acids 202—203 312—313
DIBAH = DIBAL (diisobutylaluminum hydride) see “Aluminum hydrobis(2-methylpropyl)-”
Diborane see “Borane”
Dicarbonyl type compounds see “Carboxylic acids oxo-” “Dialdehydes” “Dicarboxylic “Dioxo
Dicarboxylic acids, extended-chain, synthesis 20 37 88 328
Dicyanomethylene protecting group 166
Dicyclopropa[de,ij] naphthalene, 2a,2b,4a,4b,4c,4d-hexahydro- 332
Dieckmann condensation 55 58—59 82—83
Dieis — Alder type syntheses 81—82 84 85—86
Dieis — Alder type syntheses with hetero enes 153—154 297
Dieis — Alder type syntheses with singlet oxygen 102 276—277
Dieis — Alder type syntheses, accelerated by 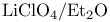 86 86
Dieis — Alder type syntheses, bridged bicyclic systems 78 85—86 92—93 210 212 331 333 337
Dieis — Alder type syntheses, domino Diels — Alder synthesis 337
Dieis — Alder type syntheses, fused bicyclo systems 80 81—82 84—86 318
Dieis — Alder type syntheses, intramolecular 84
Dieis — Alder type syntheses, intramolecular, in alkaloid synthesis 153—154 297
Dieis — Alder type syntheses, intramolecular, in steroid synthesis 280—281
Dieis — Alder type syntheses, pincer Diels — Alder synthesis 335—336
Dieis — Alder type syntheses, polar, with bulky reagents 86
Dieis — Alder type syntheses, reactivity and selectivity rules 85
Dieis — Alder type syntheses, retro- see “Retro-Diels — Alder type cleavage”
Diethylpropion,  - 302 - 302
Difunctional compounds, definition 2—3 50 147—149
Difunctional compounds, pr. 172—181 187—192
Difunctional compounds, rerro-synthetic analysis 199—207
Digitoxigenin 286—287
Dihydroxylation see “1
Diimide = diimine = 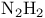 see “Diazene” see “Diazene”
Diisopropylamine etc. see “2-Propaneamine ...”
Diketones see “Dioxo compounds”
Diketopiperazines = 2,5-piperazinediones 237
Dilution see “High-dilution methods”
Dimethyl sulfoxide see “Methane sulfinylbis[-”
Dimsyl anion see “Methane sulfinylbis[- ion(1-)”
Diorganylcuprates(1-) see “Cuprates(1-) ...”
Diosgenin, Marker degradation 283—285
Diosgenin, pr. 284
Dioxygen see “Oxygen”
Diphenhydramine 300
Diphenoxylate 304
DIPHOS see “Phosphine 1
Dipyrromethanes 253—255 349
Dipyrromethenes 255—257 349
Directed aldol addition 56—62
Disconnections, retro-synthetic 194
DISIAB see “Borane bis(1
Disilazane see “Silanamine ...”
Distamycin 343—344
Distannanes, prepn. from distannoxanes 115
Distannanes, stannylation of aryl triflatcs with 295
Disulfides, diallylic, of 2-pyridinethiol, reactive S-esters from 146
Disulfides, diallylic, of thiolactams, prepn. and S-alkylation 261
Disulfides, diallylic, S extrusion from 39
Dithioacetals see “1 “1
Dithionite(2-), sodium, redn. of pyridinium 113
Divergent growth of dendrimers 354
DMAP see “4-Pyridinamine N
DMP (Dess — Martin periodinane) 134 328—329
DMSO see “Methane sulfinylbis[-”
DNA insertion (into plasmids) 243—245
DNA ligase 243—244 245—246
DNA polymerase 242 245—246
DNA synthesis, modified DNA 227 241—246 341—343 345
DNA synthesis, PCR method 226—227
Docosanedioic acid, synthesis 37
DODAC vesicles 350—351
Dodecahedrane, 1,16-dimethyl- = hexadecahydro-1,3b-dimethyl-5,2,1,6,3,4-[2,3]butanediyl-[1,4]diylidenedipentaleno[2,1,6-cde: 2',1',6'-gha]pentalene 334—337
Dodecane, 1,12-dibromo-, 1,12-diaIkylation 20
Domino — Diels — Alder reactions 209—210 337
Donor synthons 4—15
Donor synthons, 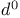 1—2 1—2
Donor synthons, 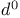 , ring opening with 69—70 76—79 , ring opening with 69—70 76—79
Donor synthons,  4—9 4—9
Donor synthons,  , reactions of 22 45—52 65—66 , reactions of 22 45—52 65—66
Donor synthons,  , stabilization of 6—9 , stabilization of 6—9
Donor synthons,  , stabilization of, by nitrogen 6 65—66 , stabilization of, by nitrogen 6 65—66
Donor synthons,  , stabilization of, by phosphorus 28—32 , stabilization of, by phosphorus 28—32
Donor synthons,  , stabilization of, by silicon 33—34 , stabilization of, by silicon 33—34
Donor synthons,  , stabilization of, by sulfur 22 45 51 66 , stabilization of, by sulfur 22 45 51 66
Donor synthons, 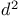 9—14 18—19 9—14 18—19
Donor synthons, 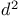 , stabilization of, by phosphorus 7 , stabilization of, by phosphorus 7
Donor synthons, 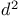 , stabilization of, by sulfur 8 , stabilization of, by sulfur 8
Donor synthons, 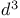 14—15 14—15
Donor synthons, 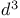 , stabilized by sulfur 14 26 , stabilized by sulfur 14 26
Donor synthons, 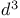 , substituted cyclopropanes 70 , substituted cyclopropanes 70
Donor synthons, 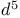 , example 15 , example 15
Donor synthons, alkyl 4—5
Donor synthons, alkyl, reactions 19—22 36—38 42—46
Donor synthons, alkyl, triorganylboranes as 9 21—22 37—38
Donor synthons, alkylidene 7—8 28—35
Donor synthons, angle of incidence to a-synthons 315—316
Donor synthons, definition 1—4
Doxycycline 317
Drugs, synthesis 299—310
Dubamine = 2-(1,3-benzodioxol-5-yl)quinoline, synthesis by Stille coupling 295
e-Synthons see “Electrocyclic reactions”
e.e. (enantiomeric excess), definition 107 footnote
E/Z-Selectivity see “cis/trans-Selectivity”
EC = (ethylamino)carbonyl 229 237
Elaidic acid = (E)-9-Octadecenoic acid 100
Electric eel acetylcholinesterase 277
Electrocyclic disconnection, retro-synthetic 194
Electrocyclic reactions (reactions of e-synthons) see “Cyclization” “Ene “Ring “Ring “Rearrangements sigmatropic”
Electrocyclizations see “Cyclizations electrocyclic”
Electron density distribution, effects on reactivity and/or regioselectivity in 1,3-dioxo 2,4-dianions 9—10 24 204
Electron density distribution, effects on reactivity and/or regioselectivity in anions of 2-en-1-ones 25
Electron density distribution, effects on reactivity and/or regioselectivity in anions of allylic thioethers 26
Electron density distribution, effects on reactivity and/or regioselectivity in anions of phosphonic diesters 29
Electron density distribution, effects on reactivity and/or regioselectivity in benzene derivs. 103—104 207—208
Electron density distribution, effects on reactivity and/or regioselectivity in dipyrromethenes 255—257
Electron density distribution, effects on reactivity and/or regioselectivity in enamines 13—14
Electron density distribution, effects on reactivity and/or regioselectivity in peroxy acids 123
Electron density distribution, effects on reactivity and/or regioselectivity in phosphonium ylides 28—29
Electronic effects, inductive see “Hyper-conjugation”
Electronic effects, steric see “Stereoelectronic control”
Electrophiles see “Acceptor synthons”
Eliminations 137—142 (see also “Decarboxylation”)
Eliminations, table 138—139
Enamines, C-acylation 88
Enamines, C-alkylation 25—26
Enamines, C-nucleophilicity 13—14
Enamines, cyclopropanation 74—75
Enamines, N-acylation 153
Enamines, N-deprotonated see “Imines N-alkyl- lithiated”
Enamines, oxidative cleavage 285
Enamines, Robinson anellation of 73 298
Enamines, synthesis by oxn. of tert. amines 138—139
Enamines, synthesis from ketones 13 88
Enantio-zeroplane, definition 359
Enantiofaces, definition 359
Enantiomeric excess (e.e.), definition 107 footnote
Enantioselective retro-synthesis 200—204
Enantioselective syntheses of  -ammo acids ( -ammo acids (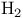 /chiral cat.) 102—103 /chiral cat.) 102—103
Enantioselective syntheses of  -branched aldehydes 22—23 25—26 -branched aldehydes 22—23 25—26
Enantioselective syntheses of  -branched carboxylic acids 22—23 -branched carboxylic acids 22—23
Enantioselective syntheses of  -branched ketones 25—26 -branched ketones 25—26
Enantioselective syntheses of  -branched ketones 20—21 -branched ketones 20—21
Enantioselective syntheses of  -hydroxy carboxylic acids by aldol type addition 59 61 62 -hydroxy carboxylic acids by aldol type addition 59 61 62
Enantioselective syntheses of  -hydroxy carboxylic acids by hydrogenation 102—103 325—326 -hydroxy carboxylic acids by hydrogenation 102—103 325—326
| Enantioselective syntheses of  -hydroxy ketones 61—62 -hydroxy ketones 61—62
Enantioselective syntheses of 1,2-diols (chiral 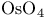 -complexes) 129 -complexes) 129
Enantioselective syntheses of 2,3-epoxy alcohols 124—127 265 321—325
Enantioselective syntheses of alcohols (with chiral hydrides) 107—108
Enantioselective syntheses of homoallylic alcohols 67—69
Enantioselective syntheses of meso-diol monoesters 167—168 276—277
Enantioselective syntheses of steroids 278—279 280 282
Enantiotopic group selectivity 126 167—168 276—277 359
Enantiotopic ligands, definition 359
endo-Peroxide of cyclic dienes 102 276—277
endo/exo-Selective reactions, acid-catd. aldol type cyclizn. 93
endo/exo-Selective reactions, alkylation with 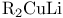 20 20
endo/exo-Selective reactions, epoxidation and glycolization 123 209—210
endo/exo-Selective reactions, [2+4]cycloadditions 78 85—86 92 209—210 297
Endonucleases, restriction- 243
Endoperoxides from 1,3-dienes 102 276—277
Ene reactions (cycloadditions with H transfer), addition of propynoic esters 282—283
Ene reactions (cycloadditions with H transfer), intramolecular, in alkaloid synth. 297—298
Ene reactions (cycloadditions with H transfer), oxidation with 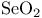 119 122 119 122
Ene reactions (cycloadditions with H transfer), rearrangement of thioethers with benzyne 39
Enol esters, oxidative cleavage 280
Enol esters, preparation 58 280
Enol ethers, addition of nitrosyl chloride 268
Enol ethers, cyclopropanation 74—75 83
Enol ethers, hydrolysis 49 103—104 278
Enol ethers, iodoetherification 272
Enol ethers, oxidative cleavage 285
Enol ethers, preparation by alcohol addn. to alkynes 64
Enol ethers, preparation by Birch reduction of alkoxyarenes 87 97 103—104 278
Enol ethers, preparation by HX-elimination of a-halo ethers 268
Enol ethers, preparation by olefination with 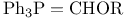 48—49 48—49
Enol ethers, preparation by Tebbe-olefination of esters 110 272
Enolate type anions, nucleophilicity differences 24—25 56
Enolate type anions, oxidation of 121—122
Enolate type anions, preparation from  -unsatd. ketones, with lithium 73 -unsatd. ketones, with lithium 73
Enolate type anions, preparation from carbonyl type compounds 9—13 22—23 26 57—58 60—63
Enolate type anions, preparation from carbonyl type compounds, E/Z-selective 12—13 22—23 26 60—62
Enolate type anions, preparation from enol esters and enol silyl ethers 24 57—58 73 281
Enolate type anions, preparation with organylcopper reagents 277
Enolate type anions, preparation with organylmagnesium reagents 281
Enolate type anions, reactions of 22—27 55—65 71—74
Enolate type anions, silylated, oxidative coupling with 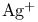 65 65
Enolate type anions, trapping with 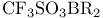 12—13 61—62 12—13 61—62
Enolate type anions, trapping with 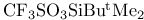 325 325
Enolate type anions, trapping with 1-propen-2-ol acetate 58
Enolate type anions, trapping with chlorotrimethylsilane 11 58 73 75 83 281
Enzymatic reactions 167 276—277
Enzyme-cleft models from Kemp’s acid 347
Ephedrine, N-methyl-, (R,R)-, chiral hydride from 107—108
Ephedrine, N-[2-(dimethylamino)ethyI]-(-)-, chiral cuprate reagents from 20—21
Ephedrine, stereoisomers, pr. 290
Ephedrine, stereoisomers, synthesis 302
Epimerization see “Racemization”
Epimerization at enolizable carbon atoms 32 78 93 265 277 283 322—323
Epimerization at enolizable carbon atoms, prevention 277 299
Epimerization of hydroxy groups by inversion-esterification 160—161 286
Epimerization of hydroxy groups via keto groups 288—323
Epimerization of tetracyclines 317
Episullldes see “Thiiranes”
Epoxides see “Oxiranes”
Equilibrium control see “Thermodynamic control”
Ergocalciferol (vitamin 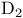 ) 289 ) 289
Ergosta-5,7,22-trien-3-ol, 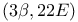 - (ergosterol), pr. 284 - (ergosterol), pr. 284
Ergosta-5,7,22-trien-3-ol, 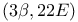 - (ergosterol), ring opening 289 - (ergosterol), ring opening 289
Ergotamine, synthesis steps 143
Erythrcmolide A seco acid, total synth. 32D—323
Erythrinane (= indolo[7a,1-a]isoquinoline) alkaloids by amine-phenol coupling 294
erythro, definition 62 360
erythro/threo-Selective syntheses see “Asymmetric induction”
erythro/threo-Selective syntheses,  -alkylation of 3-hydroxy esters 27 325—326 -alkylation of 3-hydroxy esters 27 325—326
erythro/threo-Selective syntheses, addn. of anions to oxiranes 63—64 204
erythro/threo-Selective syntheses, aldol type additions 60—62 322—323
erythro/threo-Selective syntheses, alkylation of sulfoxides 8—9
erythro/threo-Selective syntheses, allylation of carbonyl groups 66—67 325—326
erythro/threo-Selective syntheses, carbosulfenylation of alkenes 21
erythro/threo-Selective syntheses, dihydroxylation of alkenes 129
erythro/threo-Selective syntheses, epimerization via enolates 265
erythro/threo-Selective syntheses, reduction of ketones 106—107 322—323
erythro/threo-Selective syntheses, Sharpless epoxidation of allylic alcohois 125—127 265 322 324—325
Erythromycins 319
Erythronolides A and B, total synth 319—320
Eschenmoser’s principle 34—35 261
Eschenmoser’s sulfur extrusion 59—60 261
Ester condensations 55 58—59 81—83
Ester pyrolysis see “ -Eliminations” -Eliminations”
EsterilTcation see “Carboxylic acids esters”
Esters see “Carboxylic acids esters” “Sulfonic esters”
Estr-4-ene-3,17-dione, (+)-, total synth. 279
Estra-1,3,5(10)-trien-17-one,  -, synthesis 281 -, synthesis 281
Estra-1,3,5(10)-trien-17-one, 3-hydroxy- (estrone), 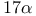 -ethynylation 62—63 -ethynylation 62—63
Estra-1,3,5(10)-trien-17-one, 3-hydroxy- (estrone), synthesis by aromatization of ring A 287—288
Estra-1,3,5(10)-triene-3,17-diol, (+)- (estradiol), total synthesis 280
Estra-1,3,5(10)-triene-3,17-diol, 17-acetate, partial synthesis 287
Estradiol, ethynyl- = 19-norpregna-1,3,5(10)-trien-20-yne-3,17-diol, 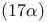 - 62—63 - 62—63
Ethanal see “Acetaldehyde”
Ethanamines, N,N-dialkyl-2-halo-, drugs from 303—304
Ethanamines, N,N-dialkyl-2-halo-, pr. 180
Ethane, nitro-,  -synthon 6 301 302 -synthon 6 301 302
Ethaneamine, N,N-diethyl- (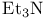 ), ),  -deprotonation of ketones 11 71 -deprotonation of ketones 11 71
Ethaneamine, N,N-diethyl- (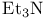 ), elimn. of HCl from ), elimn. of HCl from 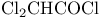 83 276 83 276
Ethaneamine, N-ethyl, Li salt (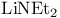 ), conversion of epoxides to allylic alcohols 27 ), conversion of epoxides to allylic alcohols 27
Ethanedioyl dichloride (oxalyl chloride), preparation of acid halides with 143
Ethanedioyl dichloride (oxalyl chloride), Swern oxidation with ClCOCOCl/DMSO 133—134 322—323 325—327
Ethaneperoxoic (peroxyacetic) acid, Baeyer — Villiger oxn. with 136 276 319—320
Ethaneperoxoic (peroxyacetic) acid, epoxidation with 34 88 123
Ethaneperoxoic (peroxyacetic) acid, trifluoro-, Baeyer — Villiger oxidation with 282
Ethaneperoxoic (peroxyacetic) acid, trifluoro-, relative reactivity 123
Ethanoic acid see “Acetic acid”
Ethanol, 2,2,2-trichloro-, phosphate protection 167 216—218 220
Ethanol, 2,2,2-trichloro-, pr. 179
Ethanol, 2-(diethylamino)- and 2-(dimethylamino), drug syntheses with 300
Ethanol, 2-(diethylamino)- and 2-(dimethylamino), pr. 177
Ethanol, 2-cyano- = 3-hydroxypropanenitrile, phosphate protection, 2-cyanoethyl ester (Ce) 166—167 216 218 220—224
Ethanol, 2-cyano- = 3-hydroxypropanenitrile, pr. 176
Ethanol, 2-[[4-(triphenylmethyl)phenyl]thio]- (TpteOH), phosphate protection 216—217
Ethanol, ion(1-) (ethanolate, ethoxide), enolization with 10 23 72—73 283
Ethanone, 1-cyclopropyi-, pr. 189
Ethanone, 1-cyclopropyi-, synthesis 130
Ethanone, 2-hydroxy-1,2-diphenyl- (benzoin), cyclic carbonate, N-prntecting reagent 164
Ethene, bromo- (vinyl bromide), cyclopropanation of 74
Ethene, bromo- (vinyl bromide), Grignard reagent from 63
Ethene, bromo- (vinyl bromide), pr. 179
Ethene, chloro-, lithiation 5
Ethene, methoxy-, cyclopropanation of 74—75
Ethene, methoxy-, pr. 174
Ethenone (ketene), 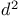 -synthon 58—59 -synthon 58—59
Ethenone (ketene), acetylation with 58—59 75
Ethenone (ketene), cyclopropanation of 75
Ethenone (ketene), dichloro- 83 276
Ethenone (ketene), dichloro-, [2+2]cycloaddn. with 83 276
Ethenone (ketene), pr. 174
Ethers see “Protection”
Ethers,  -iodo-, reductive -iodo-, reductive  -elimination 326—327 -elimination 326—327
Ethers,  -iodo-, synthesis from alkenes 272 326 -iodo-, synthesis from alkenes 272 326
Ethers, oxidation to esters or lactones 118 134—135
Ethers, synth. from esters or lactones 110—111 272
Ethoheptazine,  - 304 - 304
Ethyne, 17-ethynylation of steroids 62—63 278
Ethyne, addn. to carbonyl groups 52
Ethyne, ethoxy-, lithiated, carboxymethyl 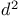 -synthon 64—65 325 327—328 -synthon 64—65 325 327—328
Etioporphyrin 256
Exhaustive dehydrogenation 139—140 338
Expansion of rings see “Ring expansion”
Extrusion of carbon dioxide 80
Extrusion of carbon monoxide 78 330
Extrusion of molecular nitrogen 35 331 334
Extrusion of sulfur 35 38—39 59—60 80 261 334 338
Extrusion of sulfur dioxide 80
Favorskii rearrangement 78 84 211
Ferrate(2-), tetracarbonyl-, acyl d-synthons from 17 46—47
|
|
 |
| Ðåêëàìà |
 |
|
|
 |
|
Î ïðîåêòå
|
|
Î ïðîåêòå



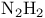 ), reduction of alkenes
), reduction of alkenes 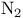 extrusion
extrusion 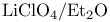
 -
- 

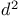
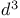
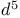 , example
, example  -ammo acids (
-ammo acids (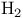 /chiral cat.)
/chiral cat.)  -branched ketones
-branched ketones 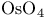 -complexes)
-complexes) 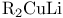
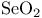
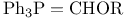
 -unsatd. ketones, with lithium
-unsatd. ketones, with lithium 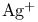
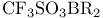
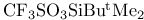
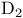 )
) 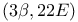 - (ergosterol), pr.
- (ergosterol), pr. 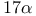 -ethynylation
-ethynylation 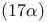 -
- 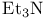 ),
), 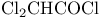
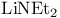 ), conversion of epoxides to allylic alcohols
), conversion of epoxides to allylic alcohols