|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. Ч Organic Synthesis: Concepts, Methods, Starting materials |

ќбсудите книгу на
Ќашли опечатку?
¬ыделите ее мышкой и нажмите Ctrl+Enter
|
Ќазвание: Organic Synthesis: Concepts, Methods, Starting materials
јвторы: Fuhrhop J., Penzlin G.
язык: 
–убрика: ’ими€/
—татус предметного указател€: √отов указатель с номерами страниц
ed2k:
»здание: second edition, rewise and enlarged
√од издани€: 1994
оличество страниц: 432
ƒобавлена в каталог: 29.10.2005
ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
|
|
 |
| ѕредметный указатель |
Acetate, piperidinium, cat. for aldol add. 82
Acetate, sodium,  -epimerization of ketones 277 -epimerization of ketones 277
Acetate, sodium, opening of oxiranes 282
Acetic acid, (dialkoxyphosphinyl)-, esters, pr. 188
Acetic acid, (dialkoxyphosphinyl)-, esters, Wittig Ч Horner olefinations 267 282
Acetic acid, anhydride (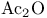 ), protection with see УProtectionФ ), protection with see УProtectionФ
Acetic acid, anhydride (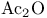 ), Pummerer rearr. with 51 265 ), Pummerer rearr. with 51 265
Acetic acid, bromo-, esters, 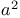 -synthon 19 65 309 -synthon 19 65 309
Acetic acid, bromo-, esters, 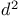 -synthon 19 301 -synthon 19 301
Acetic acid, bromo-, esters, pr. 179
Acetic acid, chloro-, esters, hydantoins from 308
Acetic acid, chloro-, esters, pr. 179
Acetic acid, cyano-, esters, pr. 177
Acetic acid, cyano-, esters, pyrrolines from 298
Acetic acid, diazo-, ethyl ester, cyclopropanes from 74Ч75
Acetic acid, diazo-, ethyl ester, pr. 176 178
Acetic acid, dichloro-, deblocking of pixyl ethers 342
Acetic acid, esters see УProtection of hydroxy groupsФ
Acetic acid, esters, 1-methylethenyl ester, pr. 174
Acetic acid, esters, 1-methylethenyl ester, transenolization 58
Acetic acid, esters, C-H acidity 10
Acetic acid, hydroxy-, esters, pr. 176
Acetic acid, hydroxyphenyl-, (S)- (L-mandelic acid), chiral auxiliary (Masamune ketone) from 62
Acetic acid, mercapto-, redn. of methionine S-oxide 229
Acetic acid, oxo-, esters, 1-nitroalkanes 301
Acetic acid, oxo-, esters, pr. 176
Acyloin coupling 53Ч54
Acyloin coupling, retro-synthetic transform 194
Acyloins see УKetones
AdamsТ hydrogenation catalyst (Pt on 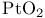 ) 105 ) 105
Adoc protective group 229
Affinity cleavage of DNA 342
AIBN see УPropanenitrile 2
Alcohols, 2-halo- see УHalohydrinsФ
Alcohols, allylic, asym. epoxidation 124Ч127 265 321Ч325
Alcohols, allylic, pr. 174
Alcohols, allylic, selective oxidation 133Ч135
Alcohols, allylic, substitution by amines [Pd] 164 303
Alcohols, allylic, synthesis, 1-alkenylation of carbonyl compds 44Ч45 62Ч63 323 326Ч327
Alcohols, allylic, synthesis, alkylation of 2-enals 303 321 322
Alcohols, allylic, synthesis, allylic oxidation of alkenes 116 119Ч123
Alcohols, allylic, synthesis, opening of oxiranes with strong bases 27
Alcohols, allylic, synthesis, reduction of 2-enones 105Ч107
Alcohols, conversions to alkenes see У -EliminationsФ -EliminationsФ
Alcohols, conversions to amines 160Ч161 267
Alcohols, conversions to halides 32 160Ч161 169 203 266 269 272 281 282
Alcohols, conversions to thiols 169
Alcohols, homoallylic, stereoselective 66Ч69 325Ч326
Alcohols, inversion-esteriflcation 160Ч161 286
Alcohols, Mitsunobu-type substitutions 160Ч161
Alcohols, oxidation to aldehydes 133Ч135
Alcohols, oxidation to carboxylic acids 134Ч135 319Ч320
Alcohols, oxidation to carboxylic esters 134Ч135
Alcohols, oxidation to ketones 109 111 133 135 266Ч267 276Ч277 283 322Ч323 328Ч329 337
Alcohols, oxidative cleavage of tert. alcohols 136
Alcohols, protection see УProtection of hydroxy groupsФ
Alcohols, protection, reduction to alkanes 97 113Ч114 203
Alcohols, synthesis from alkenes 107Ч108 117 130Ч131 283 319Ч320 325 337
Alcohols, synthesis from carbonyl compounds by alkylation 44Ч45 321Ч323
Alcohols, synthesis from carbonyl compounds by ox. cleavage 136Ч137 282 283
Alcohols, synthesis from carbonyl compounds by redn. 96 98Ч99 103Ч108 274Ч275 283 319Ч320 325 326 349
Alcohols, synthesis from carbonyl compounds by redn., enantioselective 102Ч103 108
Alcohols, synthesis from oxiranes by alkylation 21 44 269
Alcohols, synthesis from oxiranes by reduction 98Ч99 113Ч114 274Ч275 319Ч320
Alcohols, synthesis from prim, amines 202Ч203
Aldehydes with 2-enoic esters (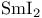 ) 69 ) 69
Aldehydes,  -alkylated 13 -alkylated 13
Aldehydes,  -alkylated, opt. active 25Ч26 -alkylated, opt. active 25Ч26
Aldehydes,  -amino-, enol ether + CINO 268 -amino-, enol ether + CINO 268
Aldehydes,  -chloro-, ketone + -chloro-, ketone + 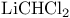 51Ч52 51Ч52
Aldehydes,  -hydroxy- 49 50 51 121Ч122 267 -hydroxy- 49 50 51 121Ч122 267
Aldehydes,  -unsaturated, ketone + -unsaturated, ketone + 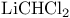 51Ч52 51Ч52
Aldehydes, 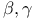 -unsaturated 70 76 -unsaturated 70 76
Aldehydes,  -hydroxy- 63Ч64 -hydroxy- 63Ч64
Aldehydes, 1-alkenylations 323
Aldehydes, 1-alkynylations 303
Aldehydes, 1-allylations 44Ч45 66Ч69 323Ч326
Aldehydes, aldol type reactions 44Ч45 56Ч58 60Ч62 322Ч323
Aldehydes, conversion to  -hydroxynitriles etc. 18 50 -hydroxynitriles etc. 18 50
Aldehydes, conversion to ketones 22 166
Aldehydes, conversion to Mannich iminium reagents 57 291Ч292
Aldehydes, conversion to sec. alcohols with RZnBr 44Ч45 301
Aldehydes, olefination 28Ч34 41 272Ч273 276 334
Aldehydes, oxidation to RCOOH or RCOOR' 134Ч135
Aldehydes, oxo- see УDioxo compoundsФ
Aldehydes, reduction to alcohols 98 105Ч106 302
Aldehydes, reduction to alkanes 98
Aldehydes, reduction to amines (via aldoximes or aldimines) 98
Aldehydes, reductive coupling 53
Aldehydes, synthesis, formylation of RX 46Ч47
Aldehydes, synthesis, hydration of 1-alkynes 117 131
Aldehydes, synthesis, hydroformylation of 1-alkenes 47Ч48
Aldehydes, synthesis, hydrolysis of 1,3-dithianes 79 328Ч329
Aldehydes, synthesis, hydrolysis of aci-nitroalkanes 16 65Ч66
Aldehydes, synthesis, oxidation of alcohols 117 133Ч135 325
Aldehydes, synthesis, oxidative cleavage of 1,2-diols 81Ч82 202 272Ч273
Aldehydes, synthesis, oxidative cleavage of alkenes 81Ч82 87Ч88 202 259 322 323 326
Aldehydes, synthesis, oxidative cleavage of oxiranes 88
Aldehydes, synthesis, oxidative rearr. of alkenes 129Ч130
Aldehydes, synthesis, Pummerer rearr. of sulfoxides 51
Aldehydes, synthesis, reduction of carboxamides 96 98 105
Aldehydes, synthesis, reduction of carboximidic esters 13 22Ч23 63Ч64 298
Aldehydes, synthesis, reduction of carboxylic anhydrides or halides 46Ч47 98
Aldehydes, synthesis, reduction of carboxylic esters 105Ч106 159 165 203 272Ч273 276 283 321 322 327Ч328 334
Aldehydes, synthesis, reduction of nitriles 50 111Ч112 298
Aldehydes, synthesis, reductive formylation of ketones 48Ч49
Aldehydes, umpolung 17 51
Aldol-type reactions 55Ч62 81Ч83 328Ч329
Aldol-type reactions, acid-catalyzed 55Ч59 81 93 208 279
Aldol-type reactions, diastereoselective 60Ч62 322Ч323
Aldol-type reactions, directed 55Ч62
Aldol-type reactions, enantioselective 59 61Ч62
Aldol-type reactions, retro- see УRetro-aldol type cleavageФ
Alkali metals, redn. with see УBirch reductionФ
Alkaloids 289Ч299
Alkaloids, pr. 290
Alkaloids, syntheses, electrocyclic reactions 153Ч154 295Ч299
Alkaloids, syntheses, Mannich/Vilsmeier type reactions 291Ч293
Alkaloids, syntheses, phenol and aryl coupling 293Ч295
Alkanes, branched, carbosulfenylation of alkenes 21
Alkanes, branched, hydromethylation of alkenes 105
Alkanes, halo- see УHaloalkanesФ
Alkanes, oxidation 118Ч119 285Ч286
Alkanes, synthesis by reduction by reductive cleavage of nitriles 280
Alkanes, synthesis by reduction by reductive coupling, of alkenes 37
Alkanes, synthesis by reduction by reductive coupling, of halides 36
Alkanes, synthesis by reduction of alcohols 97 113Ч114 203
Alkanes, synthesis by reduction of alkenes 96Ч97 101Ч105
Alkanes, synthesis by reduction of arenes 103Ч104
Alkanes, synthesis by reduction of carbonyl groups 98 109
Alkanes, synthesis by reduction of cyclopropanes 76 105
Alkanes, synthesis by reduction of halides 83 114Ч115 275 287 319Ч320 335Ч336
Alkenes (carbon-carbon double bonds), acidity of allylic C-H 9Ч10
Alkenes (carbon-carbon double bonds), alkoxy- see УEnol ethersФ
Alkenes (carbon-carbon double bonds), carbosulfenylation (addn. of У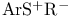 Ф) 21 Ф) 21
Alkenes (carbon-carbon double bonds), conversion to aldehydes, ketones and tert. alcohols with borane + CO 9 47Ч48
Alkenes (carbon-carbon double bonds), coupling with organyl halides 42Ч43
Alkenes (carbon-carbon double bonds), double-bond shift 85 90Ч91 101 274 278
Alkenes (carbon-carbon double bonds), E/Z isomerizn 31 100Ч102 282 296 332
Alkenes (carbon-carbon double bonds), E/Z isomerizn via trans-glycol thiocarbonates 142
Alkenes (carbon-carbon double bonds), halo- (vinyl halides), from alkynes 132 325
Alkenes (carbon-carbon double bonds), hydration via boranes 107Ч108 130Ч131 319Ч320 325 337
Alkenes (carbon-carbon double bonds), hydration with mercury (2+) 283
Alkenes (carbon-carbon double bonds), hydromethylation 105
Alkenes (carbon-carbon double bonds), oxidation 117 123Ч130
Alkenes (carbon-carbon double bonds), oxidation to 1,2-dihalides 84 119 123Ч124 156Ч157
Alkenes (carbon-carbon double bonds), oxidation to 1,2-diols 123Ч124 127Ч130 276Ч277
Alkenes (carbon-carbon double bonds), oxidation to 1,3-dienes 123Ч124 333Ч334
Alkenes (carbon-carbon double bonds), oxidation to halohydrins 77 117 275Ч276 286Ч287
Alkenes (carbon-carbon double bonds), oxidation to halolactones 275 319Ч321 335Ч336
| Alkenes (carbon-carbon double bonds), oxidation to oxiranes by epoxidation 18 123Ч127 156Ч157 265 321Ч325
Alkenes (carbon-carbon double bonds), oxidation to oxiranes via halohydrins 77 117 287 319Ч320
Alkenes (carbon-carbon double bonds), oxidative cleavage 81Ч82 87Ч89 259 280 282 322 323 326
Alkenes (carbon-carbon double bonds), oxidative rearrangement 129Ч130
Alkenes (carbon-carbon double bonds), protection see УProtection of carbon-carbon double bondsФ
Alkenes (carbon-carbon double bonds), reduction with 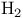 /cat 96Ч97 101Ч103 278 283 /cat 96Ч97 101Ч103 278 283
Alkenes (carbon-carbon double bonds), reduction with (Z)-diazene 102
Alkenes (carbon-carbon double bonds), reduction with alkali metals 103Ч104 278
Alkenes (carbon-carbon double bonds), reduction with boranes 89Ч90 97 130Ч131
Alkenes (carbon-carbon double bonds), sterically hindered, snythesis 33Ч35 41
Alkenes (carbon-carbon double bonds), sulfonation with 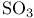 252 252
Alkenes (carbon-carbon double bonds), synthesis 28Ч44 (see also У -EliminationsФ) -EliminationsФ)
Alkenes (carbon-carbon double bonds), synthesis, cis-stereoselective 28Ч31 37 64 100Ч101
Alkenes (carbon-carbon double bonds), synthesis, olefination of carbonyl groups 28Ч35 110 125 267 272Ч273 276 281Ч283 334
Alkenes (carbon-carbon double bonds), synthesis, ox. dimerization of P ylides 31
Alkenes (carbon-carbon double bonds), synthesis, reduction of alkynes 40 64 100Ч101
Alkenes (carbon-carbon double bonds), synthesis, reductive coupling of alkynes 37Ч38
Alkenes (carbon-carbon double bonds), synthesis, reductive dimerization of carbonyl groups 34Ч35 41
Alkenes (carbon-carbon double bonds), synthesis, Tebbe methylenation of esters 110
Alkenes (carbon-carbon double bonds), synthesis, umpolung 18 211
Alkyl anions 4Ч5
Alkyl halides see УHaloalkanesФ
Alkyl synthons see УAcceptor synthonsФ УDonor
Alkynes see У1-AlkynesФ
Alkynes, acid-catalysed cyclization 91Ч92 279Ч280
Alkynes, alcohol addition to 64
Alkynes, hydration via boranes 117 131
Alkynes, hydration, with mercury(2+) 52 57 117
Alkynes, hydroboration and coupling 37Ч38
Alkynes, hydrobromination/hydrozirconation 132 325
Alkynes, oxidation or ox. cleavage 117 132
Alkynes, reduction to alkenes with 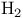 [catalyst] 40 64 96Ч97 100Ч101 155 [catalyst] 40 64 96Ч97 100Ч101 155
Alkynes, reduction to alkenes with DIBAL 100
Alkynes, reduction to alkenes with Li or Na 96Ч97 100Ч101
Alkynes, synthesis, alkynylation of RX 36 64 325
Alkynes, synthesis, fragmentation of hydrazones 89 142
Alkynes, synthesis, methylidynation with 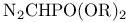 325 325
Allyl a-synthons 22 27 299 303 325Ч326
Allyl d-synthons 66Ч69 90
Aluminate(1-), hydrotris(2-methyl-2-propanolato)-, redn. with 47Ч48 107
Aluminate(1-), tetrahydro-, lithium, + ![$\mathrm{AlCl_{3}} (3:4) [\rightarrow\mathrm{AlH_{3}}]$](/math_tex/f21c6ea6cdc993d6f728e184dde5095482.gif) , redn. with, reduction with, of , redn. with, reduction with, of  -unsaturated ketones 105Ч106 -unsaturated ketones 105Ч106
Aluminate(1-), tetrahydro-, lithium, + ![$\mathrm{AlCl_{3}} (3:4) [\rightarrow\mathrm{AlH_{3}}]$](/math_tex/f21c6ea6cdc993d6f728e184dde5095482.gif) , redn. with, reduction with, of pyridines 112Ч113 , redn. with, reduction with, of pyridines 112Ч113
Aluminate(1-), tetrahydro-, lithium, chiral reducing agents from 107Ч108
Aluminate(1-), tetrahydro-, lithium, reduction with, of alkyl tosylates 114
Aluminate(1-), tetrahydro-, lithium, reduction with, of carboxamides 112Ч113 247 291
Aluminate(1-), tetrahydro-, lithium, reduction with, of carboxylic esters 325 326 328 349
Aluminate(1-), tetrahydro-, lithium, reduction with, of ketones 106Ч107 322Ч323
Aluminate(1-), tetrahydro-, lithium, reduction with, of nitriles 50 298
Aluminate(1-), tetrahydro-, lithium, reduction with, of oxiranes 114
Aluminate(1-), tris(alkanolato)hydro-, prepn. 96
Aluminate(1-), tris(alkanolato)hydro-, redn. with 98 107Ч108
Aluminum amalgam (Al-Hg),  -deoxygenation of -deoxygenation of  -hydroxy ketones 277 -hydroxy ketones 277
Aluminum chloride (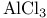 ), Lewis acid catalyst for aldol type reactions 81 ), Lewis acid catalyst for aldol type reactions 81
Aluminum chloride (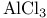 ), Lewis acid catalyst for alkyne cyclotrimerization 330 ), Lewis acid catalyst for alkyne cyclotrimerization 330
Aluminum chloride (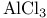 ), Lewis acid catalyst for Friedel Ч Crafts reactions 15Ч16 291 ), Lewis acid catalyst for Friedel Ч Crafts reactions 15Ч16 291
Aluminum chloride (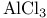 ), Lewis acid catalyst for glycosyl chloride synthesis 266 ), Lewis acid catalyst for glycosyl chloride synthesis 266
Aluminum chloride (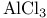 ), Lewis acid catalyst for redn. with ), Lewis acid catalyst for redn. with 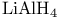 105Ч106 112Ч113 105Ч106 112Ч113
Aluminum hydride (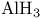 ) 105Ч106 112Ч113 ) 105Ч106 112Ч113
Aluminum oxide (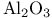 ), basic cat. 32 ), basic cat. 32
Aluminum tris(2-propanolate), Oppenauer oxn. with 117 133Ч134 278
Aluminum(1+), diethyl-, sulfate (![$\mathrm{[Et_{2}Al]_{2}SO_{4}}$](/math_tex/5c1aba954aeda9617d0c5143a50e31af82.gif) ), catalyst for carbonyl allyl-stannylation 66Ч67 ), catalyst for carbonyl allyl-stannylation 66Ч67
Aluminum, chlorodiethyl- (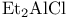 ), catalyst for alkynylation of oxiranes 64Ч65 ), catalyst for alkynylation of oxiranes 64Ч65
Aluminum, chlorodiethyl- (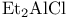 ), catalyst for УeneФ reaction of 2-alkynoic esters 282 ), catalyst for УeneФ reaction of 2-alkynoic esters 282
Aluminum, hydrobis(2-methylpropyl)- (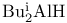 , DIBAL), reduction with, 4-methoxybenzylidene acetals 326 , DIBAL), reduction with, 4-methoxybenzylidene acetals 326
Aluminum, hydrobis(2-methylpropyl)- (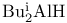 , DIBAL), reduction with, alkynes 97 100 , DIBAL), reduction with, alkynes 97 100
Aluminum, hydrobis(2-methylpropyl)- (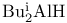 , DIBAL), reduction with, carboxamides 98 , DIBAL), reduction with, carboxamides 98
Aluminum, hydrobis(2-methylpropyl)- (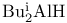 , DIBAL), reduction with, carboxylic esters 105Ч106 282 283 334 , DIBAL), reduction with, carboxylic esters 105Ч106 282 283 334
Aluminum, hydrobis(2-methylpropyl)- (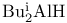 , DIBAL), reduction with, lactones 105 159 165 203 273 276 321 322 , DIBAL), reduction with, lactones 105 159 165 203 273 276 321 322
Aluminum, hydrobis(2-methylpropyl)- (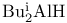 , DIBAL), reduction with, nitriles 50 96 111Ч112 , DIBAL), reduction with, nitriles 50 96 111Ч112
Amfepramone, (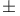 )- 302 )- 302
Amidation see УCarboxamidesФ
Amide, bis(trimethylsilyl)- see УSilanamine ...Ф
Amide, diisopropyl- see У2-Propanamine ...Ф
Amides, alkali, basicity 5 10
Amides, alkali, deprotonation with, of acidic C-H 10 300 303Ч304
Amides, alkali, of 1-alkynes 5 36 64
Amides, alkali, of haloalkanes 74Ч75 92
Amines, allylic or benzylic, reductive cleavage 97Ч98 113Ч114
Amines, allylic or benzylic, synthesis 164 303
Amines, protection see УProtectionФ
Amines, synthesis by reduction of carboxamides 98 111Ч112 247
Amines, synthesis by reduction of imines 112Ч113
Amines, synthesis by substitution of allylic acetates 164
Amino sugars 263 267Ч268
Ammonia, deblocking with, of O-acyl derivatives 157Ч158
Ammonia, deblocking with, of protected DNA 224 342
Amphiphiles, synthetic 269 350Ч356
Ampicillin 311
ancat = anti-catenoid, definition 360
Androst-4-erie-3,17-dione, (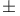 )- 280 )- 280
Androst-5-en-17-one, 3-(acetyloxy)-, (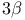 )- (andro-stenolone acetate), partial synth. 118Ч119 )- (andro-stenolone acetate), partial synth. 118Ч119
Anellations see УCarbocyclesФ
Angle of incidence in nucleophilic addns 315Ч316
Anisoles see УArenes alkoxy-Ф
Annealing of DNA primers 227
Annealing of sticky ends 225Ч226 243Ч244
Annulenes, bridged 38 123Ч124 333Ч334
Anthracene, 9-(chloromethyl)-, mutagen 341Ч342
Anthracene, Diels Ч Alder addn. to 93
Anti/syn-selectivity see Уcis/trans-selectivityФ Уerythro/threo-selectivityФ
Antibiotics, synth. 310Ч329 (see also УGriseofulvinsФ)
Antithesis 171 193
Arabinose DЧ and LЧ pr 263
Arborol 355Ч356
Arenes, alkoxy-, reduction 87 97 103Ч104
Arenes, electrophilic substitution at 207Ч208 305Ч306 309
Arenes, nucleophilic substitution at 42Ч43 305 310
Arenes, redn., with 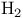 /catalyst 87 112Ч113 /catalyst 87 112Ч113
Arenes, redn., with Li or Na 87 97 103Ч104
Arenes, synth. by dehydrogenation 139Ч140 338
Arnstein tripeptide 313Ч314
Aromadendrene 32
Aromadendrene, cyclopropa[a]naphthalene precursor 85
Artemisia alcohol 45
Aspartic acid, pr. 184
Aspartic acid, protection 229
Aspirin 301
Asymmetric induction see УEnantioselectiveФ
Asymmetric induction, chiral ketones 62 106Ч107
Asymmetric induction, chiral sulfoxides 8Ч9
Asymmetric induction, steroid synthesis 27 278Ч281
Asymmetric syntheses see УEnantioselective ...Ф
Asymmetry of vesicle membranes 351
Atropisomers, 5,10,15,20-tetraarylporphyrins 253
Atropisomers, KempТs acid arylimides 347
Atropisomers, porphyrin oligomers 348Ч349
Atropisomers, УbinapФ chelands 102Ч103
Axial/equatorial stereoselectivity, 1-alkylation of cyclic ketones with 1-alkynes 63
Axial/equatorial stereoselectivity, 1-alkylation of cyclic ketones with Cu and Zn organyls 44Ч45
Axial/equatorial stereoselectivity, 1-alkylation of cyclic ketones with sulfur ylides 45
Axial/equatorial stereoselectivity, 2-alkylation of cyclic enamines 13 25Ч26 73
Axial/equatorial stereoselectivity, 2-alkylation of cyclic hydrazones 12
Axial/equatorial stereoselectivity, 3-alkylation of cyclic enones 20 72
Axial/equatorial stereoselectivity, dihydroxylation of cycloalkenes 127Ч129
Axial/equatorial stereoselectivity, epimerization of alcohols 288
Axial/equatorial stereoselectivity, reduction of ketones 105Ч107
Azepines, hexahydro- (azepanes) 149 304
Azides, acyl- see УCarboxylic acids azidesФ
Azides, heterocycles from 153Ч154
Azides, reduction to amines 267
Azines, olefins from 35
Aziridines, alkene + amine, 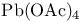 212Ч213 212Ч213
Aziridines, alkene + amine, cyclization of 2-halo amines 154
Aziridines, alkene + amine, thermal decomposition of azides 154
Azlactones see У5(4H)-OxazolonesФ
Azodicarboxylic acid see УDiazenedicarboxylic acidФ
Bacteria, genetic engineering with 241Ч246
Baeyer Ч Villiger rearrangement 80 136Ч137 165 210 275Ч276 282 283 319Ч320
Baldwin rules 315Ч316
Barbaralone = ![$tricyclo[3.3.1.0^{2,8}]nona-3,6-dien-9-one$](/math_tex/d0b3a8147b848c9adc6b7a82fca5ff1482.gif) 94 94
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 -epimerization of ketones
-epimerization of ketones 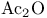 ), protection with
), protection with 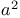 -synthon
-synthon 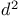 -synthon
-synthon 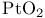 )
)  -EliminationsФ
-EliminationsФ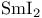 )
) 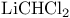
 -unsaturated, ketone +
-unsaturated, ketone + 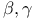 -unsaturated
-unsaturated  -hydroxy-
-hydroxy- 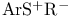 Ф)
Ф) 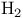 /cat
/cat 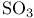
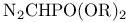
![$\mathrm{AlCl_{3}} (3:4) [\rightarrow\mathrm{AlH_{3}}]$](/math_tex/f21c6ea6cdc993d6f728e184dde5095482.gif) , redn. with, reduction with, of
, redn. with, reduction with, of 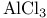 ), Lewis acid catalyst for aldol type reactions
), Lewis acid catalyst for aldol type reactions 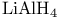
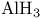 )
) 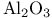 ), basic cat.
), basic cat. ![$\mathrm{[Et_{2}Al]_{2}SO_{4}}$](/math_tex/5c1aba954aeda9617d0c5143a50e31af82.gif) ), catalyst for carbonyl allyl-stannylation
), catalyst for carbonyl allyl-stannylation 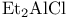 ), catalyst for alkynylation of oxiranes
), catalyst for alkynylation of oxiranes 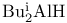 , DIBAL), reduction with, 4-methoxybenzylidene acetals
, DIBAL), reduction with, 4-methoxybenzylidene acetals 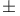 )-
)- 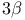 )- (andro-stenolone acetate), partial synth.
)- (andro-stenolone acetate), partial synth. 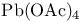
![$tricyclo[3.3.1.0^{2,8}]nona-3,6-dien-9-one$](/math_tex/d0b3a8147b848c9adc6b7a82fca5ff1482.gif)