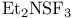|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. Ч Organic Synthesis: Concepts, Methods, Starting materials |

ќбсудите книгу на
Ќашли опечатку?
¬ыделите ее мышкой и нажмите Ctrl+Enter
|
Ќазвание: Organic Synthesis: Concepts, Methods, Starting materials
јвторы: Fuhrhop J., Penzlin G.
язык: 
–убрика: ’ими€/
—татус предметного указател€: √отов указатель с номерами страниц
ed2k:
»здание: second edition, rewise and enlarged
√од издани€: 1994
оличество страниц: 432
ƒобавлена в каталог: 29.10.2005
ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
|
|
 |
| ѕредметный указатель |
Phosphorous triamide, hexamethyl-, Mitsunobu type reactions with 161
Phosphorous tribromide, OH/Br exchange 303
Phosphorous trichloride, glycosyl halides 266
Phosphorus(5+) chloride, amide cleavage with 311Ч313
Phosphorus(5+) chloride, prepn. of acid halides with 143
Phosphorus(5+) oxide (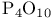 ), Bischler Ч Napieralski isoquinoline synth. 293 ), Bischler Ч Napieralski isoquinoline synth. 293
Phosphoryl chloride (phosphorus oxychloride), Vilsmeier reagents from 16 Ч255 293
Photochemical reactions, 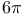 -electrocyclizations 295Ч296 338 -electrocyclizations 295Ч296 338
Photochemical reactions, 1,3-cyclohexadiene opening 289
Photochemical reactions, decomposition of  -diazo ketones 337 -diazo ketones 337
Photochemical reactions, decomposition of acyl hypoiodites 337
Photochemical reactions, decomposition of azides 154
Photochemical reactions, E/Z isomerizn. of alkenes 31 282 296 332
Photochemical reactions, elimination of 1,2-dicarboperoxoic esters 142
Photochemical reactions, extrusion of 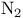 334 334
Photochemical reactions, extrusion of CO 330 339
Photochemical reactions, extrusion of sulfur (with 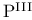 ) 39 ) 39
Photochemical reactions, oxidation of alkyl groups 116 285Ч286
Photochemical reactions, skeletal rearr. of strained polycycles 329Ч333
Photochemical reactions, [2+2]cycloaddition, alkene + alkene 78 297Ч298 330 333
Photochemical reactions, [2+2]cycloaddition, alkene + oxygen (ox. cleavage) 259
Photochemical reactions, [2+2]cycloaddition, benzene + benzene 336Ч337
Photochemical reactions, [2+2]cycloreversion 333
Photochemical reactions, [2+4]cycloaddition, photooxygenation, diene + oxygen 102 276Ч277
Photosynthetic reaction centre, models 348Ч350
Phthaloyl protecting group 163 315
Picted Ч Spengler cyclization 292
Pig pancreatic lipase 167
Pinacol coupling 53
Pinacolone rearrangement of 1,2-diol monotosylate 32
Pinane derivs., pr. 192
Pincer Diels Ч Alder addition 335Ч336
Piperazine, asymm. 1,4-dialkylation 304
Piperazine, industrial synthesis 149
Piperazine-2,5-diones (diketopiperazines)- by products in peptide synthesis 237
Piperidine derivs., synthesis 304
Piperidine, enamines from 13Ч14
Piperidine-2,6-diones, synthesis 304
Piperidinium acetate, cat. for aldol addn 82
Pivalaldehyde, amino acid N,O-protection 299
Pivampicillin 312
Pixyl protecting group 341Ч342
Plasmids, vehicles for gene transfer 242Ч246
Platinum, hydrogenation catalyst 105 109 113
Platinum, rearrangement of carbon cages with 336
Platyphyllide, retro-synthesis 208
PMB (p-methoxybenzyl) ethers 157 326Ч329
Polycyclic carbon skeletons, syntheses 3 90Ч94 208Ч212 329Ч339 356Ч357
Polymerase chain reaction 226Ч227
Polymeric supports see УSolid-phase synthesisФ
Polystyrene derivs. see УBenzene ethenyl- homopolymerФ
Porphobilinogen, cyclotetramerization 251Ч252
Porphodimethenes 255
Porphyrin oligomers, arylene-linked, stacked, stretchei and tilted 348Ч350
Porphyrin, УcappedФ 253
Porphyrin, Уpicked-fenceФ 253
Porphyrinogens 250Ч252
Porphyrins 250Ч257
Porphyrins, 5,10,15,20-tetrasubstituted 252Ч253
Porphyrins, assemblies, covalent/non-covalent 348Ч350
Porphyrins, micellar fibers (amphiphilic porphyrins) 350
Porphyrins, natural, total synthesis 251Ч252 255Ч257
Porphyrins, numbering 252
Porphyrins, reduction of 251 258Ч259
Porphyrins, unsymmetrical 256Ч257
Porphyrins, water-soluble 252
Potassium(1+), complex or organic salts (except organyls) see corresponding acid or anion
Potassium, (triphenylmethyl)- (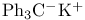 ), enolate formation with 58 ), enolate formation with 58
Potassium, preparation of Ti(0) with 41
PPY see УPyridine 4-(1-pyrrolidinyl)-Ф
Pregn-4-ene-3,20-dione (progesterone),  -, total synthesis 279Ч280 -, total synthesis 279Ч280
Pregn-4-ene-3,20-dione (progesterone), pr. 284
Previtamin 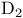 289 289
Previtamin 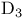 , , 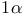 -hydroxy-, synthesis 34 -hydroxy-, synthesis 34
Prevost glycolization 127
prim. Amines, addn. of to 2-enones 291
prim. Amines, addn. of to 2-enones to oxiranes 291
prim. Amines, conversion to alcohols 202Ч203
prim. Amines, oxidation to nitrenes, 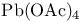 212Ч213 212Ч213
prim. Amines, synthesis by degradn. of carboxylic acid derivs. 139 143 301 308 331
prim. Amines, synthesis by reduction of azides 267
prim. Amines, synthesis by reduction of nitriles 50 98
prim. Amines, synthesis by reduction of nitroalkanes 60 98 112 253 297
prim. Amines, synthesis by reduction of nitrosoalkanes 268
prim. Amines, synthesis from alcohols 267
Primers for DNA synthesis by PCR 226Ч227
Primers, mismatch-, for single-site mutation 245Ч246
Prismane = ![$tetracyclo[2.2.0.0^{2,6}.0^{3,5}]hexane$](/math_tex/31dd65f3b121860f501aedba6650bf9482.gif) (Ladenburg benzene) 330Ч331 (Ladenburg benzene) 330Ч331
Prochirality, terminology 167 359Ч360
Prochlorperazine 310
Promethazine,  - 310 - 310
Propadrine,  - 302 - 302
Propanal, 2,2-dimethyl- (pivalaldehyde), N,O-protection of proline 299
Propane, 1,3-dibromo-, alkylation with 23
Propane, 1-bromo-3-chloro-, drug synthesis with 304 310
Propane, 1-bromo-3-chloro-, pr. 180
Propane, 2-isocyano-2-methyl- ( ), diallylnickel trapping with 41 ), diallylnickel trapping with 41
Propanedinitrile, carbon acidity 10
Propanedinitrile, protecting reagent 166
Propanedioic acids, esters, alkylation 23 44 199Ч200
Propanedioic acids, esters, allylation with  -allylpalladium complexes 27 -allylpalladium complexes 27
Propanedioic acids, esters, carbon acidity 10
Propanedioic acids, esters, carboxymethyl 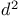 -synthon 19 139 199Ч200 -synthon 19 139 199Ч200
Propanedioic acids, esters, drugs from 302 305Ч306
Propanedioic acids, esters, Knoevenagel condensation with 56
Propanedioic acids, esters, Michael addition with 72
Propanedioic acids, esters, pr. 177
Propanenitrile, 2,2'-azobis[2-methyl- (AIBN, 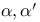 -azoisobutyronitrile), radical initiator 111 -azoisobutyronitrile), radical initiator 111
Propanenitrile, 2-hydroxy-2-methyl- (acetone cyanohydrin), educt 201
Propanenitrile, 2-hydroxy-2-methyl- (acetone cyanohydrin), pr. 176
Propanenitrile, 3-hydroxy-, phosphate protection, 2-cyanoethyl (Ce) esters 166Ч167 216 218 220Ч221 224
Propanenitrile, 3-hydroxy-, pr. 176
Propanoic acid, 2-bromo-, ethyl ester, pr. 179
Propanoic acid, 2-bromo-, ethyl ester, Reformatsky reaction with 44Ч45
Propanoyl chloride, 2-(acetyloxy)-2-methyl- (MoffattТs reagent)-, chlorohydrins from diols 160 327Ч328
Propanoyl chloride, 2-(acetyloxy)-2-methyl- (MoffattТs reagent)-, O-protection 160
Propionic acid see УPropanoic acidФ
Prostaglandins 273Ч277
Prostaglandins, cyclopentane precursor 159 210 275Ч277
Prostaglandins, partial synthesis 274Ч275
Prostaglandins, total syntheses 275Ч277
Protection 154Ч167 (see also УCappingФ УDeblockingФ)
Protection of  -methylene groups of acetoacetic esters, deprotonation 9 24 204 326 -methylene groups of acetoacetic esters, deprotonation 9 24 204 326
Protection of  -methylene groups of ketone, arylmethylene derivatives 82 -methylene groups of ketone, arylmethylene derivatives 82
Protection of  -methylene groups of ketone, butylthiomethylene derivatives 155Ч156 -methylene groups of ketone, butylthiomethylene derivatives 155Ч156
Protection of  -methylene groups of ketone, thioketals 79 155Ч156 -methylene groups of ketone, thioketals 79 155Ч156
Protection of 1-alkynes, deprotonation 100Ч101
Protection of 1-alkynes, silylation 155 339
Protection of amino acids, N,O-alkylidene derivs. 299
Protection of amino groups, amides and carbamates 161Ч164 216 220Ч221 228Ч229 304 327 342
Protection of amino groups, complexation with metal ions 293Ч294
Protection of amino groups, oxazolones (УOxФ derivs.) 161 164
Protection of amino groups, phthalimides 163
Protection of amino groups, quaternization 293 304
Protection of amino groups, sulfonamides 247 304
Protection of carbon-carbon double bonds, 1,2-dihalides 119 156Ч157
Protection of carbon-carbon double bonds, oxiranes 156Ч157
Protection of carbonyl groups, acetals 18 20 24 49 75 104 106 122 135 165Ч166 279 291 298 337 339 349
Protection of carbonyl groups, dicyanomethylene derivatives 166
Protection of carbonyl groups, dithioacetals 76 165Ч166
Protection of carbonyl groups, monothioacetals 76 165Ч166
Protection of carbonyl groups, olefination 321Ч323
Protection of carboxy groups, 2,2,2-trihaloethyl esters 165
Protection of carboxy groups, esters 32 37 47Ч48 165 229 307
Protection of carboxy groups, silyl esters 313
Protection of diols, cyclic acetals 158 209Ч210 263Ч269 271 272 276Ч277 318 321Ч323 326
Protection of enols, (1,1-dimethylethyl)dimethylsilyl ethers 325
Protection of enols, acetic esters 58 318
Protection of enols, dialkylborinic esters 12Ч13 60Ч62
Protection of enols, ethers 48Ч49 103Ч104 278
| Protection of enols, trimethylsilyl ethers 11 53Ч54 57Ч58 73 75 83 86 209Ч210 281
Protection of guanidino groups 229
Protection of hydroxy groups 157Ч161 (see also УMonosaccharidesФ УNucleotidesФ)
Protection of hydroxy groups, carbonic and carboxylic esters 55 75 157Ч158 160Ч161 216 266Ч273 276Ч277 282 283
Protection of hydroxy groups, ethers, (1,1-dimethylethyl)dimethylsilyl (Tbdms, Tbs, 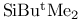 ) 34 65 67 69 131 157 159 160 276Ч277 281Ч282 320Ч325 327Ч329 ) 34 65 67 69 131 157 159 160 276Ч277 281Ч282 320Ч325 327Ч329
Protection of hydroxy groups, ethers, (1,1-dimethylethyl)diphenylsilyl 110 272
Protection of hydroxy groups, ethers, (4-methoxyphenyl)methyl (MPM, PMB) 157 270 325Ч329
Protection of hydroxy groups, ethers, 1,1-dimethylethyl (tert-butyl, 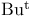 ) 229 280 283 ) 229 280 283
Protection of hydroxy groups, ethers, 2,4,4-trimethyl-5-oxo-1,3-dioxolan-2-yl (Moffat dioxolanone) 160
Protection of hydroxy groups, ethers, 2-propenyl (allyl) 270
Protection of hydroxy groups, ethers, 9-phenyl-9H-xanthen-9-yl (pixyl, Px) 341Ч342
Protection of hydroxy groups, ethers, diphenylmethyl (benzhydryl, 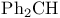 ) 265 ) 265
Protection of hydroxy groups, ethers, methoxymethyl (MOM) 84 318
Protection of hydroxy groups, ethers, methyl, irreversible 23 157 161 272
Protection of hydroxy groups, ethers, methyl, reversible 280
Protection of hydroxy groups, ethers, phenylmethyl (benzyl, Bzl, Bn,  ) 131 157Ч159 161 229 241 270Ч272 301 305 328Ч329 ) 131 157Ч159 161 229 241 270Ч272 301 305 328Ч329
Protection of hydroxy groups, ethers, tetrahydro-2ff-pyran-2-yl (Thp) 26 82 157 159Ч160 201 216 273
Protection of hydroxy groups, ethers, triethylsilyl ( ) 328Ч329 ) 328Ч329
Protection of hydroxy groups, ethers, trimethylsilyl ( , Tms) 14 15 70 75 83 159 275 281Ч283 315 , Tms) 14 15 70 75 83 159 275 281Ч283 315
Protection of hydroxy groups, ethers, triphenylmethyl (trityl, Tr), p-methoxytrityl (Mtr), diЧpЧmethoxytrityl (Dmtr) 159Ч160 216 218Ч224 343
Protection of hydroxy groups, ethers, tris(1-methylethyl)silyl (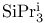 ) 324Ч329 ) 324Ч329
Protection of hydroxy groups, iodoetheriflcation 272 326Ч327
Protection of hydroxy groups, lactones 319Ч320 321Ч322 327Ч328
Protection of mercapto groups 169 229 237Ч240
Protection of nucleic acid sequences 344
Protection of phenols, methyl ethers 280
Protection of phenols, phenylmethyl (benzyl) ethers 301 305
Protection of phosphate groups 166Ч167 216Ч224
Protection of phosphate groups, 1-methyl-2-oxopropyl (acetoinyl, Acn) 219
Protection of phosphate groups, 2,2,2-trichloroethyl (Tce) 167 216 220
Protection of phosphate groups, 2-cyanoethyl (Ce) 166Ч167 216 220Ч224
Protection of phosphate groups, 2-[[4-(triphenylmethyl)phenyl]thio]ethyl (Tpte) 216 217
Protection of phosphate groups, 4-chlorophenyl 218
Protection of phosphate groups, methyl (Me) 220Ч224
Protection of thioether groups, sulfoxides 229
Pseudodiosgenin 285
Pseudoephedrine,  - 302 - 302
Pseudoionone, cyclization 90Ч91
PTC = phase-transfer catalysts see УBenzene methanaminiumФ УButamiminiumФ У18-Crown-6Ф
Pulegone see УCyclohexanone 5-methyl-2-(1-methylethylidene)-Ф
Pummerer rearrangements 51 265
Px, pixyl protecting group 341Ч342
Pyrene, 10b,10c-dihydro-10b,10c-dimethyl-, trans- 38
Pyridine, 2,6-bis(1,1-dimethylethyl)-, bulky base 321
Pyridine, 2,6-bis(1,1-dimethylethyl)-, hydrochloride, kinetic protonation with 323
Pyridine, 2,6-dimethyl-, bulky base 223 322 329
Pyridine, 4-(1-pyrrolidinyl)- (PPY), acylation catalyst 145 326Ч327
Pyridine, attenuation of 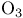 -reactivity 326 -reactivity 326
Pyridines, 2-halo-, 2-alkylation 303Ч304
Pyridines, redn. 112Ч113 212 297 303 309
Pyridinium ions, nucleophilic alkylation 309
Pyridinium ions, reduction 112Ч113 212 297
Pyridinium, 2-chloro-1-methyl-, iodide (MukaiyamaТs macrolactamization agent) 328Ч329
Pyridinium, chlorochromate see УChromate(1-)Ф
Pyrrolidine, 2-(methoxymethyl)- (S)-, chiral ligand 20Ч21
Pyrrolidine, enamines from 13Ч14
Pyrrolo[1,2-a]pyrimidine, 2,3,4,6,7,8-hexahydro- (1,5-diazabicyclo[4.3.0]non-5-ene, DBN), HBr elimination with 334
Pyrromethanes see УDipyrromethanesФ
Pyrromethenes see УDipyrromethenesФ
Quaternary ammonium see УAmmoniumФ
quaternary Ammonium compounds, Hofmann elimination 72 140Ч141 331
quaternary Ammonium compounds, phase-transfer catalysts see УBenzenemethanaminiumФ У1-ButanaminiumФ
quaternary Ammonium compounds, thermal demethylation 304
Quinidine, 10,11-dihydro-, chiral 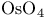 -catalyst 129 -catalyst 129
Quinidine, pr. 290
Quinine, 10,11-dihydro-, chiral 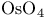 -catalyst 129 -catalyst 129
Quinine, pr. 290
Quinoiines, synthesis by Heck coupling 43
Quinones see УCyclohexadienedionesФ
Quinuclidine derivs. = 1-azabicyclo[2.2.2]octanes, drug synthesis 309
Quinuclidine derivs. = 1-azabicyclo[2.2.2]octanes, pr. 290
Racemates, resolution of see УOptical resolutionФ
Racemization, prevention in  -lactam formation 161 -lactam formation 161
Racemization, prevention in peptide synthesis 145 231Ч232 234
Radical synthons, 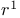 , ketyl radical anions 53Ч54 , ketyl radical anions 53Ч54
Radical synthons, 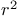 , enol radicals 65 , enol radicals 65
Radical synthons, 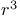 , allyl radicals 41Ч42 84 , allyl radicals 41Ч42 84
Radical synthons, alkyl r 20 35 36
Radical synthons, alkynyl 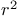 40 40
Radicals in autoxidation of enolates 121
Radicals in oxidation of alkyl groups 286
Radicals in photoisomerizations 331Ч333
Radicals in reduction with tin hydrides 110Ч111
Raney 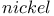 (activated Ni-Al alloy), catalytic hydrogenation of arenes 87 (activated Ni-Al alloy), catalytic hydrogenation of arenes 87
Raney 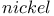 (activated Ni-Al alloy), catalytic hydrogenation of nitroalkenes 66 112 (activated Ni-Al alloy), catalytic hydrogenation of nitroalkenes 66 112
Raney 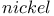 (activated Ni-Al alloy), cleavage of 1,3-oxathiolanes 165Ч166 (activated Ni-Al alloy), cleavage of 1,3-oxathiolanes 165Ч166
Raney 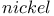 (activated Ni-Al alloy), reductive desulfurization of sulfones 70 (activated Ni-Al alloy), reductive desulfurization of sulfones 70
Raney 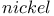 (activated Ni-Al alloy), reductive desulfurization of thioethers, thiols and dithioacetals 76 82 98 109 156 273 (activated Ni-Al alloy), reductive desulfurization of thioethers, thiols and dithioacetals 76 82 98 109 156 273
Rearrangement, retro-synthetic 195
Rearrangements see УRing contractionФ УRing УRing
Rearrangements via carbenes 75 92 141Ч142
Rearrangements, acid-base-catalysed, of alkenes ( shift) 85 90Ч91 101 274 278 shift) 85 90Ч91 101 274 278
Rearrangements, acid-base-catalysed, of O-acyl isoureas 144 234
Rearrangements, base-induced, of tosylhydrazones 141Ч142
Rearrangements, base-induced, of triorganylborane adducts 9 37Ч38
Rearrangements, cationic see УRearrangements oxidativeФ
Rearrangements, cationic, of  -(methylsulFinyl) ketones (Pummerer rearr.) 51 265 -(methylsulFinyl) ketones (Pummerer rearr.) 51 265
Rearrangements, cationic, of  -chloro thioethers 314 -chloro thioethers 314
Rearrangements, cationic, of 1,2-diols (pinacolone rearr.) 32
Rearrangements, cationic, of 2,5-cyclohexadien-1-ones 139
Rearrangements, cationic, of glycosides with 2-OH by 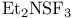 272 272
Rearrangements, cationic, of ketones etc. with 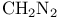 83 166 83 166
Rearrangements, cationic, of N-halo amines (Stieglitz rearr.) 77Ч78
Rearrangements, cationic, of oxiranes 52 77 79
Rearrangements, cationic, of porphyrinogens and dipyrromethanes 251Ч252 254
Rearrangements, metal-catalysed, of alkenes, H shift 90 101 270
Rearrangements, metal-catalysed, of allyl alcohols to aldehydes 43
Rearrangements, metal-catalysed, of oligocycloalkanes 79Ч80 332Ч333 336
Rearrangements, oxidative, of 1-alkenyl boranes 37Ч38
Rearrangements, oxidative, of 1-amino alcohols 76Ч77
Rearrangements, oxidative, of alkenes to carbonyl compds 129Ч130
Rearrangements, oxidative, of carbonyl compounds by peroxy acids see УBaeyer Ч Villiger rearrangementФ
Rearrangements, oxidative, of ketones by thallium(3+) 136
Rearrangements, oxidative, of oximes to amides (Beckmann rearr.) 136Ч137
Rearrangements, reductive, of diallylic disulfides 39
Rearrangements, sigmatropic see УPericyclic reactionsФ
Rearrangements, sigmatropic, [1,16]-, H shift in corrin synthesis 262
Rearrangements, sigmatropic, [1,2]-, of sulfonium ylides (Stevens rearr.) 38 338
Rearrangements, sigmatropic, [1,3]-, of (1-alkenyl)cyclopropanes 77 83
Rearrangements, sigmatropic, [1,3]-, of cyclopropanemethanimines 298Ч299
Rearrangements, sigmatropic, [1,5]-, H shift in alkaloid synthesis 295
Rearrangements, sigmatropic, [1,7]-, H shift of tachysterols 289
Rearrangements, sigmatropic, [2,3]-, in oxn. with 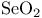 119 122 119 122
Rearrangements, sigmatropic, [2,3]-, of allylsulfonium ylides 39
Rearrangements, sigmatropic, [2,3]-, of diallyl thioethers with benzyne 39
Rearrangements, sigmatropic, [3,3]-, in FischerТs indole synthesis 151Ч152
Rearrangements, sigmatropic, [3,3]-, of 6-allyl-2,4-cyclohexadien-1-ones 319Ч320
Rearrangements, transamidations, УzipФ reaction 249Ч250
Recombinant DNA 241Ч246
Reduction 96Ч115 (see also corresponding compound classes УBirch У reductiveФ УHydrogenationФ)
Reduction of  -(methylsulfinyl) ketones 51 -(methylsulfinyl) ketones 51
Reduction of  -hydroxy ketones 156 277 -hydroxy ketones 156 277
Reduction of acid anhydrides or halides to aldehydes 46Ч47
Reduction of alkenes 96Ч97 101Ч104 130Ч131
Reduction of alkynes 64 96Ч97 100Ч102
Reduction of carbonyl compds 98 105Ч111 141Ч142
Reduction of carbonyl compds, enantioselective 108
Reduction of carboximidic esters to aldehydes 13 22Ч23 63Ч64 298
Reduction of carboximidic esters to sec. amines 111Ч112
Reduction of haloalkanes 97 113Ч115 202Ч203
Reduction of haloalkanes with chromium(2+) 286
Reduction of haloalkanes with hydrogen/Raney Ni 287
Reduction of haloalkanes with methyllithium 75
Reduction of haloalkanes with tributylstannane 97 114Ч115 275 319Ч320
Reduction of haloalkanes with zinc or zinc-copper 83 335Ч336
Reduction of hydroperoxides 121
Reduction of nitrogen compounds 111Ч113
Reduction of peroxides 277
Reduction, selectivity of reagents, tables 96Ч99
Reductive  -climination see У -climination see У -EliminationФ -EliminationФ
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



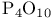 ), Bischler Ч Napieralski isoquinoline synth.
), Bischler Ч Napieralski isoquinoline synth. 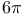 -electrocyclizations
-electrocyclizations  -diazo ketones
-diazo ketones 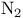
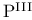 )
) 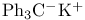 ), enolate formation with
), enolate formation with  -, total synthesis
-, total synthesis 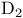
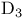 ,
, 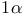 -hydroxy-, synthesis
-hydroxy-, synthesis 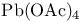
![$tetracyclo[2.2.0.0^{2,6}.0^{3,5}]hexane$](/math_tex/31dd65f3b121860f501aedba6650bf9482.gif) (Ladenburg benzene)
(Ladenburg benzene)  ), diallylnickel trapping with
), diallylnickel trapping with  -allylpalladium complexes
-allylpalladium complexes 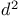 -synthon
-synthon 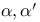 -azoisobutyronitrile), radical initiator
-azoisobutyronitrile), radical initiator 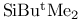 )
) 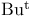 )
) 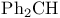 )
)  )
)  )
)  , Tms)
, Tms) 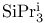 )
) 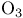 -reactivity
-reactivity 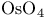 -catalyst
-catalyst -lactam formation
-lactam formation 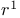 , ketyl radical anions
, ketyl radical anions 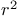 , enol radicals
, enol radicals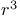 , allyl radicals
, allyl radicals 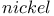 (activated Ni-Al alloy), catalytic hydrogenation of arenes
(activated Ni-Al alloy), catalytic hydrogenation of arenes 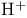 shift)
shift)