|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. Ч Organic Synthesis: Concepts, Methods, Starting materials |

ќбсудите книгу на
Ќашли опечатку?
¬ыделите ее мышкой и нажмите Ctrl+Enter
|
Ќазвание: Organic Synthesis: Concepts, Methods, Starting materials
јвторы: Fuhrhop J., Penzlin G.
язык: 
–убрика: ’ими€/
—татус предметного указател€: √отов указатель с номерами страниц
ed2k:
»здание: second edition, rewise and enlarged
√од издани€: 1994
оличество страниц: 432
ƒобавлена в каталог: 29.10.2005
ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
|
|
 |
| ѕредметный указатель |
Iodine = diiodine (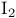 ), ),  isomerization of polyenes 31 isomerization of polyenes 31
Iodine = diiodine (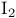 ), iodoetherification with 272 326 ), iodoetherification with 272 326
Iodine = diiodine (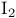 ), iodolactonization with 321 335Ч336 ), iodolactonization with 321 335Ч336
Iodine = diiodine (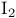 ), oxidative cleavage of organylzirconium 132 ), oxidative cleavage of organylzirconium 132
Iodine, phenylbis(trifluoroacetato)- + methanol (Stork reagent), 1,3-dithiane cleavage 328Ч329
Iodoalkanes see УHaloalkanesФ
Iodoetherification 272 326
Iodolactones see УHalolactonesФ
Ion-exchange resins see УBenzene ethenyl-Ф УSulfonic
Ionone,  - or - or  -, pr. 191 -, pr. 191
Ionone,  - or - or  -, synthesis 90Ч91 -, synthesis 90Ч91
Ionone, 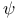 -, acid-catalysed cyclization of 90Ч91 -, acid-catalysed cyclization of 90Ч91
Ipc (isopinocampheyl), chiral auxiliary group 108
Ireland Ч Claisen rearrangement 87 325
Iron(1+),  -, addition of carbon nucleophiles 44 -, addition of carbon nucleophiles 44
Iron(1+),  -, addition of carbon nucleophiles 44 -, addition of carbon nucleophiles 44
Iron, 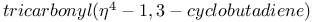 - 78 - 78
Iron,  -, coupling with alkenes 44 -, coupling with alkenes 44
Iron,  -, ox. coupling with carbon nucleophiles 44 -, ox. coupling with carbon nucleophiles 44
Iron, anionic carbonyl complexes see УFerrate ...Ф
Isobutyryl chloride,  -acetoxy- 160 -acetoxy- 160
Isocyanates, blocking of OH with 301
Isocyanates, blocking of SH with 229 238
Isocyanates, synthesis 143 301 331
Isocyanides, trapping of diallylnickel 41
Isodrin, pagodane and dodecahedrane from 336Ч337
Isodrin, pr. 192
Isoephedrine,  - 302 - 302
Isomerization, configurational,  and/or and/or  of alkenes via 1,2-diol thiocarbonates 142 of alkenes via 1,2-diol thiocarbonates 142
Isomerization, configurational,  and/or and/or  of alkenes with hydrogenation catalyst 100Ч102 of alkenes with hydrogenation catalyst 100Ч102
Isomerization, configurational,  and/or and/or  of alkenes, photochemical 31 282 296 332 of alkenes, photochemical 31 282 296 332
Isomerization, configurational,  and/or and/or  of alkenes, thermal or iodine-catalysed 31 of alkenes, thermal or iodine-catalysed 31
Isomerization, configurational, cis/trans or  at rings see УEpimerizationФ at rings see УEpimerizationФ
Isomerization, constitutional see УRearrangementsФ
Isopinocampheyl, chiral auxiliary group 108
Isoprene see У1 2-methyl-Ф
Isopropamide iodide,  - 302 - 302
Isoquinolines 292Ч293
Isoquinuclidine derivs. = 2-azabicyclo[2.2.2]-octane derivs., retro-synthesis 212Ч213
Isoquinuclidine derivs. = 2-azabicyclo[2.2.2]-octane derivs., synthesis 153 291 297
Isothioureas, S-alkyl-, thiols from 168
Isoureas, O-acyl- = carbamimidic anhydrides, reactive intermediates 144Ч145 234
Isoureas, O-alkyl- = carbamimidic esters 144Ч145
Isoxazoles 307Ч308
Isoxazoles, 4,5-dihydro- 153
Isoxazolidines 153
Jasmone = (Z)-3-methyl-2-(2-pentenyl)-2-cyclopenten-1-one 29
JonesТ reagent see УChromium(6+) oxide with sulfuric acidФ
Juglone = 5-hydroxy-1,4-naphthalenedione, dienophilc in tetracycline synthesis 318
Julia Ч Lythgoe olefination 34
JuliaТs terpenoid synthesis 69Ч70
Juvenile hormone, synth. steps 20 30 135 155
KAPA see У1 mono-K
Katsuki Ч Sharpless oxn. see УSharpless epoxidationФ
Kekulene = 15,23:16,22-dimethenobenzo[1,2-a:5,4-a']dipentaphene 338
KempТs acid 346Ч347
KempТs acid, molecular complexes of derivatives 346Ч348
Ketenes see УEthenoneФ
Ketimines see УIminesФ
Ketones,  -(alkylsulflnyl)-, synthesis and conversions 50Ч51 65 -(alkylsulflnyl)-, synthesis and conversions 50Ч51 65
Ketones,  -acidity of 10 -acidity of 10
Ketones,  -activation of see УEnaminesФ УEnolatesФ УVinylogous -activation of see УEnaminesФ УEnolatesФ УVinylogous
Ketones,  -acylation of 81 -acylation of 81
Ketones,  -acylation of, via enamines 88 -acylation of, via enamines 88
Ketones,  -acylation of, via sulfur extrusion 59Ч60 -acylation of, via sulfur extrusion 59Ч60
Ketones,  -acylthic, S extrusion from 59Ч60 -acylthic, S extrusion from 59Ч60
Ketones,  -alkoxy-, by oxn. of alkynes 117 132 -alkoxy-, by oxn. of alkynes 117 132
Ketones,  -alkylation of 24Ч26 82 87 -alkylation of 24Ч26 82 87
Ketones,  -amino-, pyrroles from 150Ч151 -amino-, pyrroles from 150Ч151
Ketones,  -amino-, synthesis from enol ethers 268 -amino-, synthesis from enol ethers 268
Ketones,  -amino-, synthesis from ketones 147 151 302 -amino-, synthesis from ketones 147 151 302
Ketones,  -halo-, -halo-, 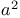 -synthons 2 16 18 -synthons 2 16 18
Ketones,  -halo-, -halo-,  -alkylation with C nucleophiles 63 -alkylation with C nucleophiles 63
Ketones,  -halo-, -halo-,  -amination 302 -amination 302
Ketones,  -halo-, Favorskii rearr. of 78 84 -halo-, Favorskii rearr. of 78 84
Ketones,  -halo-, reduction to oxiranes, enantioselective 108 -halo-, reduction to oxiranes, enantioselective 108
Ketones,  -hydroxy- (acyloins), Birch type a-deoxygenation 156 277 -hydroxy- (acyloins), Birch type a-deoxygenation 156 277
Ketones,  -hydroxy- (acyloins), conversion to imidazoles 151Ч152 -hydroxy- (acyloins), conversion to imidazoles 151Ч152
Ketones,  -hydroxy- (acyloins), synthesis of, by oxidation of alkynes 132 -hydroxy- (acyloins), synthesis of, by oxidation of alkynes 132
Ketones,  -hydroxy- (acyloins), synthesis of, by oxidation of ketones 116 121Ч122 -hydroxy- (acyloins), synthesis of, by oxidation of ketones 116 121Ч122
Ketones,  -hydroxy- (acyloins), synthesis of, by redn. of 1,2-diones with -hydroxy- (acyloins), synthesis of, by redn. of 1,2-diones with  219 219
Ketones,  -hydroxy- (acyloins), synthesis of, from carbonyl compounds with 1,3-dithiane anions 51 -hydroxy- (acyloins), synthesis of, from carbonyl compounds with 1,3-dithiane anions 51
Ketones,  -hydroxy- (acyloins), synthesis of, from carbonyl compounds with 1-alkyne anions 52 -hydroxy- (acyloins), synthesis of, from carbonyl compounds with 1-alkyne anions 52
Ketones,  -hydroxy- (acyloins), synthesis of, from carboxylic acid esters by acyloin coupling 53Ч54 -hydroxy- (acyloins), synthesis of, from carboxylic acid esters by acyloin coupling 53Ч54
Ketones,  -hydroxy- (acyloins), synthesis of, from carboxylic acid esters with DMSO anion 51 -hydroxy- (acyloins), synthesis of, from carboxylic acid esters with DMSO anion 51
Ketones,  -dehydrogenation of 138Ч140 -dehydrogenation of 138Ч140
Ketones,  -unsaturated, -unsaturated,  - and - and 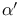 -alkylation of 24Ч25 82 277 -alkylation of 24Ч25 82 277
Ketones,  -unsaturated, -unsaturated,  , p-dialkylation 277 , p-dialkylation 277
Ketones,  -unsaturated, -unsaturated,  -alkylation of 20Ч21 277 -alkylation of 20Ч21 277
Ketones,  -unsaturated, conversion to cyclobutanones 75/77 -unsaturated, conversion to cyclobutanones 75/77
Ketones,  -unsaturated, conversion to cyclopentanones 75/77 83 -unsaturated, conversion to cyclopentanones 75/77 83
Ketones,  -unsaturated, Favorskii rearr. of 84 211 -unsaturated, Favorskii rearr. of 84 211
Ketones,  -unsaturated, hydration of 274Ч275 -unsaturated, hydration of 274Ч275
Ketones,  -unsaturated, oxidative cleavage of 82 -unsaturated, oxidative cleavage of 82
Ketones,  -unsaturated, reduction of, to alkenes 109 -unsaturated, reduction of, to alkenes 109
Ketones,  -unsaturated, reduction of, to allylic alcohols 105Ч107 -unsaturated, reduction of, to allylic alcohols 105Ч107
Ketones,  -unsaturated, reduction of, to enolates with Li 73 -unsaturated, reduction of, to enolates with Li 73
Ketones,  -unsaturated, reduction of, to enolates with RMgBr 281 -unsaturated, reduction of, to enolates with RMgBr 281
Ketones,  -unsaturated, reduction of, to ketones with -unsaturated, reduction of, to ketones with 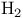 /catalyst 283 /catalyst 283
Ketones,  -unsaturated, reduction of, to ketones with Li 73 103Ч104 -unsaturated, reduction of, to ketones with Li 73 103Ч104
Ketones,  -unsaturated, synthesis of, by -unsaturated, synthesis of, by  -dehydrogenation of ketones 122 138Ч140 -dehydrogenation of ketones 122 138Ч140
Ketones,  -unsaturated, synthesis of, by -unsaturated, synthesis of, by  -eliminations 57 138 140 274 -eliminations 57 138 140 274
Ketones,  -unsaturated, synthesis of, by -unsaturated, synthesis of, by  -eliminations of sulfoxides 65 86 -eliminations of sulfoxides 65 86
Ketones,  -unsaturated, synthesis of, by aldol type condensations 59 71Ч73 81 82 280 -unsaturated, synthesis of, by aldol type condensations 59 71Ч73 81 82 280
Ketones,  -unsaturated, synthesis of, by Horner oleflnation 29 -unsaturated, synthesis of, by Horner oleflnation 29
Ketones,  -unsaturated, synthesis of, by Mannich reactions 57 -unsaturated, synthesis of, by Mannich reactions 57
Ketones,  -unsaturated, synthesis of, by oxidation of allylic -unsaturated, synthesis of, by oxidation of allylic 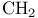 116 119Ч120 116 119Ч120
Ketones,  -unsaturated, synthesis of, by oxidation of allylic alcohols 118 133Ч135 323 -unsaturated, synthesis of, by oxidation of allylic alcohols 118 133Ч135 323
Ketones,  -unsaturated, synthesis of, by reduction of alkoxyarenes 278 -unsaturated, synthesis of, by reduction of alkoxyarenes 278
Ketones,  -amino- (Mannich bases), -amino- (Mannich bases),  -elimination of 57 72Ч73 -elimination of 57 72Ч73
Ketones,  -amino- (Mannich bases), pr. 178 -amino- (Mannich bases), pr. 178
Ketones,  -amino- (Mannich bases), synthesis 57 300 304 -amino- (Mannich bases), synthesis 57 300 304
Ketones,  -hydroxy-, synthesis by aldol type addn 55Ч58 60Ч62 -hydroxy-, synthesis by aldol type addn 55Ч58 60Ч62
Ketones,  -hydroxy-, synthesis by hydration of 2-enones 274Ч275 -hydroxy-, synthesis by hydration of 2-enones 274Ч275
Ketones, 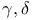 -unsaturated, synth. by Torgov reaction 71 278Ч279 -unsaturated, synth. by Torgov reaction 71 278Ч279
Ketones, (triphenylphosphoranylidene)hydrazones, Barton olefination of thiones with 35
Ketones, 1,2-oxygen shift procedure 156
Ketones, 1-alkenylation of 62Ч63
Ketones, 1-alkylation of 44
Ketones, 1-alkynylation of 52 62Ч63 278
Ketones, 1-allylation of 66Ч69
Ketones, conversion of, to  -chloro aldehydes ( -chloro aldehydes (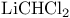 ) 51Ч52 ) 51Ч52
Ketones, conversion of, to  -hydroxy aldehydes (TosMIC) 49 51 -hydroxy aldehydes (TosMIC) 49 51
Ketones, conversion of, to aldehydes ( ) 48Ч49 ) 48Ч49
Ketones, conversion of, to cyclopropanol silyl ethers 75 83
Ketones, conversion of, to enamines 13 88
Ketones, conversion of, to enol silyl ethers 11 58 75 83
Ketones, conversion of, to enolates 11Ч12 24Ч25 58
Ketones, conversion of, to nitriles (TosMIC) 49
Ketones, conversion of, to oxiranes (sulfonium ylides) 28 45
Ketones, cyclic, 5-membered, synthesis 81Ч83
Ketones, cyclic, selective monoalkylation 25Ч26
Ketones, cyclic, synthesis, Birch redn. of alkoxyarenes 103Ч104 278
Ketones, cyclic, synthesis, from dienes and  47Ч48 47Ч48
Ketones, homologization of, by diazoalkanes 83 166
Ketones, olefination 28Ч35 41 110 267
Ketones, oxidative a-coupling via enol ethers 65
Ketones, oxidative cleavage of, Beckmann rearr. to lactams 136Ч137
Ketones, oxidative cleavage of, by  via enol ethers 285 via enol ethers 285
Ketones, oxidative cleavage of, by 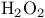 via via  -(arylmethylene) ketones 82 -(arylmethylene) ketones 82
Ketones, oxidative cleavage of, by 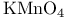 137 137
Ketones, oxidative cleavage of, by fragmentation via a-dithioketals 79
Ketones, oxidative cleavage of, to esters or lactones 80 136 165 206 210 276 282 283 319Ч320
Ketones, protection of see УProtection of carbonyl groupsФ
Ketones, rearrangements via tosylhydrazones 141Ч142
Ketones, reduction of, to alcohols 98 105Ч108
Ketones, reduction of, to alcohols, enantiosel 102Ч103 108 325Ч326
Ketones, reduction of, to alkanes 98 109
Ketones, reduction of, to alkenes 141Ч142
Ketones, reduction of, to amines via imines or oximes 98 112
| Ketones, reductive coupling of 35 41 53
Ketones, reductive coupling of, with 2-enoic esters 69
Ketones, synthesis of, form aldehydes via 1,3-dithianes 22 51
Ketones, synthesis of, form aldehydes via dicyanomethylene derivs. 166
Ketones, synthesis of, form alkenes via boranes + CO 47Ч48
Ketones, synthesis of, form alkenes, ox. cleavage 87Ч88 156Ч157 280 282
Ketones, synthesis of, form alkenes, ox. rearr. with  129Ч130 129Ч130
Ketones, synthesis of, from 5(4H)-oxazolones + 2RX 48
Ketones, synthesis of, from arf-nitroalkanes, aq. acid 16 65Ч66
Ketones, synthesis of, from carboxylic acid derivs.,  46Ч47 46Ч47
Ketones, synthesis of, from carboxylic acid derivs., halides, 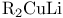 or RZnX 45Ч46 or RZnX 45Ч46
Ketones, synthesis of, from carboxylic acid derivs., lithium salts, RLi 45Ч46 282
Ketones, synthesis of, from carboxylic acid derivs., nitriles, RMgX 201 298Ч299
Ketones, synthesis of, from carboxylic acid derivs., thioesters, RLi or RMgX 319Ч320
Ketones, synthesis of, from diallylic Ni complexes + RNC 41
Ketones, synthesis of, from dithioacetals + RX 22 51 66 205
Ketones, synthesis of, from enol or enamine derivatives, hydrolysis 48Ч49 103Ч104 278
Ketones, synthesis of, from enol or enamine derivatives, ox. cleavage with  or or 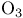 280 285 280 285
Ketones, synthesis of, from hydrazones, 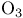 26 26
Ketones, synthesis of, from nitroalkenes, reduction 301
Ketones, synthesis of, from sec. alcohols by oxidation 133Ч135 267 276Ч277 283 322Ч323 328Ч329 337
Ketones, synthesis of, from tetracarbonylferrate(2-)/CO 46Ч47
Ketones, synthesis of, from thioenol ethers by hydrolysis 83
Kinetic control, diastereotoposelective lactonization 168
Kinetic control, directed aldol addns. 56Ч62
Kinetic control, enolate formation 11Ч13 24Ч25 58
Kinetic control, Wittig olefination 28Ч31
Kinetic protonation of enolates 322Ч323
Kinetic resolution see УOptical resolutionФ
Knoevenagel condensation 56 58Ч59 166
KnorrТs pyrrole synthesis 150Ч151
Koenigs Ч Knorr glycosylation with glycosyl halides and mercury(2+) 267
L-Arabinitol, 1,2:4,5-dianhydro-, prepn. 160 327Ч328
L-Arabinitol, chiral educt 160 327Ч328
L-Arginine, pr. 184
L-Arginine, protection 229
L-Cysteine, cephalosporin from 313Ч314
L-Cysteine, pr. 185
L-Cysteine, S-protection 229
L-Glutamic acid, chiral educt 202Ч203
L-Glutamic acid, pr. 184
L-Glutamic acid, protection 229
L-Hexoses, total synthesis 264Ч265
L-Homoproline, FK 506 synthon 324 326Ч327
L-Mandelic acid = (S)-hydroxyphenylacetic acid, chiral auxiliary from 62
L-Proline, chiral ligand from 20Ч21
L-Proline, N,O-protection 299
L-Proline, stereoselectivity  -alkylation 299 -alkylation 299
L-Serine,  -lactams from serine amides 161 -lactams from serine amides 161
L-Serine, educt for chiral auxiliary 23
L-Serine, pr. 184
L-Serine, protection 229
L-Streptose, synthesis 267
L-Valinol = (S)-2-amino-3-methyl-butanol, chiral auxiliary 61
Lactams, macrocyclic, synthesis 240Ч241 247 249Ч250 328Ч329
Lactams, pr. 178 184
Lactams, synth. from cyclic ketoximes 136Ч137 195
Lactones,  -alkylation, diastereoselective 321 322 327Ч328 -alkylation, diastereoselective 321 322 327Ч328
Lactones, macrocyclic, synthesis by intramol. allyl coupling 42
Lactones, macrocyclic, synthesis by intramol. esterification 145Ч146
Lactones, reduction to cyclic ethers 110Ч111
Lactones, reduction to cyclic hemiacetals 105 321 322 327Ч328
Lactones, synthesis by Baeyer Ч Villiger oxidation 80 136 165 206 210 276 319Ч320
Lactones, synthesis by halolactonization 275 319Ч320 321
Lactones, synthesis by intramol. esterification 63Ч65 69 70 145Ч146 168 273 322 325 327Ч328
Ladenburg benzene = prismane 330Ч331
Lanosta-8,24-dien-3-ol,  - (lanosterol), biosynthesis, syntheses relating to 279Ч280 - (lanosterol), biosynthesis, syntheses relating to 279Ч280
Lanosta-8,24-dien-3-ol,  - (lanosterol), pr. 284 - (lanosterol), pr. 284
Lawesson type reagents 110Ч111
LDA see У2-Propaneamine N-(1-methylethyl)- lithium
Lead(2+) acetate, additive for Lindlar cat 100
Lead(2+) acetate, basic acetate,  , catalyst for aldol type condensation 318 , catalyst for aldol type condensation 318
Lead(4+) acetate, oxidation with, of alcohols to aldehydes 117 133
Lead(4+) acetate, oxidation with, of amines to nitrenes 212Ч213
Lead(4+) acetate, oxidation with, of hydrazines to diazenes 35
Lead(4+) acetate, oxidative degradation of 1,2-dicarboxylic acids 80 142 333
Lead(4+) acetate, oxidative degradation of a-amino acids 48
Lead(4+) acetate, oxidative degradation of carboxylic acids with  337 337
Lecithins, vesicle membranes 350
Leukotriene 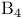 , synthesis 125Ч126 , synthesis 125Ч126
Lewis acids see УAluminum(3+) chlorideФ УBorane trifluoro-Ф УLithium(1+)Ф УMagnesium(2+)Ф УMercury(2+)Ф УSilvei(1+)Ф УThallium(1+)Ф УTin(4+) УTitanium(4+) УZinc(2+)Ф
Librium 307
Ligands, macrocyclic 235Ч237 241 247Ч262
Lindlar catalyst 100
Lindlar catalyst, hydrogenation of alkynes 40 64 100Ч101
Linear synthesis vs. convergent synthesis, disadvantages 224Ч225 233Ч234
Lipase, pig pancreatic 167
Lipid membranes 350Ч351
Lithium(1+), complex or organic salts (except organyls) see corresponding acid or anion
Lithium(1+), perchlorate, cat. for Diels Ч Alder addition 86
Lithium(1+), salts, cat. for dehydrogenation 52 140
Lithium(1+), salts, cat. for rearr. of oxiranes 52 77 79
Lithium, (1,1-dimethylethyl)- ( ), halogen-metal exchange with 70 326Ч327 ), halogen-metal exchange with 70 326Ч327
Lithium, (1,1-dimethylethyl)- ( ), lithiation with, of sulfones 8 ), lithiation with, of sulfones 8
Lithium, (1,1-dimethylethyl)- ( ), lithiation with, of thioesters 323 ), lithiation with, of thioesters 323
Lithium, (1,1-dimethylethyl)- ( ), prepn. of ), prepn. of 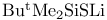 from 169 from 169
Lithium, (1,3-dithian-2-yl)- see У1
Lithium, (1-methylpropyl)- ( ), lithiation of allylic CH groups 14 15 ), lithiation of allylic CH groups 14 15
Lithium, (2-methoxycyclopropyl)-, cis-, 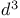 -synthon 14Ч15 19 70 76 -synthon 14Ч15 19 70 76
Lithium, (2-methoxycyclopropyl)-, cis-, prepn. 70
Lithium, (ethoxyethynyl)-, carboxymethyl 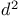 -synthon 64Ч65 325 327Ч328 -synthon 64Ч65 325 327Ч328
Lithium, (triphenylmethyl)- (trityllithium,  ), selective enolization with 11Ч12 ), selective enolization with 11Ч12
Lithium, alkyl-, addn. to 1-aikenyl silanes 34
Lithium, alkyl-, addn. to lithium carboxylates 45Ч46 283
Lithium, alkyl-, addn. to thionolactones 110Ч111
Lithium, butyl- (BuLi) 5
Lithium, butyl- (BuLi), lithiation with, of 1-alkynes 64Ч65 303 325 328
Lithium, butyl- (BuLi), lithiation with, of 1-alkynes, 1,3-dilithiation 281
Lithium, butyl- (BuLi), lithiation with, of allylic and benzylic CH groups 10 14
Lithium, butyl- (BuLi), lithiation with, of carboxylic acids (dilithiation) 63
Lithium, butyl- (BuLi), lithiation with, of dithioacetals 6 8 22 51 66
Lithium, butyl- (BuLi), lithiation with, of imidic esters 22Ч23
Lithium, butyl- (BuLi), lithiation with, of phosphonic diamides 328 329
Lithium, butyl- (BuLi), lithiation with, of phosphonium salts 6 31Ч32
Lithium, butyl- (BuLi), lithiation with, of sec. carboxamides 61
Lithium, butyl- (BuLi), lithiation with, of silanes (activated) 7
Lithium, butyl- (BuLi), lithiation with, of sulfones 34
Lithium, butyl- (BuLi), lithiation with, of sulfonium salts 45 154
Lithium, butyl- (BuLi), lithiation with, of tosylhydrazones 141
Lithium, dichloromethyl, addn. to ketones 51Ч52
Lithium, ethenyl- (vinyllithium) 5
Lithium, ethynyl-, addition to oxiranes 321
Lithium, metalation of organyl halides with 5Ч6
Lithium, methyl-, alkyl d-synthon 1Ч2 79 283
Lithium, methyl-, cleavage of silyl enolates with 57Ч58 73
Lithium, methyl-, halogen-metal exchange with 74Ч75
Lithium, methyl-, lithiation with, of sulfoxides 9
Lithium, methyl-, lithiation with, of tosylhydrazones 141
Lithium, methyl-, metal-metal exchange, with Cu(1+) 5 20
Lithium, methyl-, transmetalation of organylstannanes 322Ч323
Lithium, reduction with see УBirch reductionФ
Lithium, reductive desulfurization of sulfones 70
Lithium, [(methylthioXtrimethylsilyl)methyl]-, Peterson olefination with 34
Lithium-biphenyl reagent, 10-demethylation of steroids 287Ч288
Lithium-naphthalene reagent, deprotonation of 1-alkynes 52
Lysine, pr. 184
Lysine, protection 229 241
Macrocycles see УCarbocycles macrocyclicФ
Macrocycles, lactams 247 249Ч250 324Ч329
Macrocycles, lactones 42 145Ч146 319Ч329
Macrocycles, ligands 235Ч236 247 249Ч250
Macrocycles, peptides 235Ч236 241
Macrocycles, peptides, solid-phase method 241
Macrolides see УLactones macrocyclicФ
Magnesium(2+) halides, catalysts, for allylstannane addn. to RR'CO 66Ч67
Magnesium(2+) halides, catalysts, for cyclopropanemethanol opening 70 77
Magnesium(2+) halides, transmetalation of organyllithium 326Ч327
Magnesium, alkylhalo-, metal-metal exchange of 5 7 20 46
Magnesium, alkylhalo-, preparation 7 20 46 326Ч327
Magnesium, bromoethenyl-, ethenylation with 18 62Ч63
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



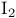 ),
),  isomerization of polyenes
isomerization of polyenes  - or
- or  -, pr.
-, pr. 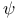 -, acid-catalysed cyclization of
-, acid-catalysed cyclization of  -, addition of carbon nucleophiles
-, addition of carbon nucleophiles  -, addition of carbon nucleophiles
-, addition of carbon nucleophiles 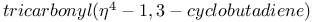 -
-  -, coupling with alkenes
-, coupling with alkenes  -
-  and/or
and/or  at rings
at rings 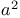 -synthons
-synthons 
 -dehydrogenation of
-dehydrogenation of 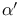 -alkylation of
-alkylation of 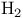 /catalyst
/catalyst 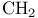
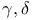 -unsaturated, synth. by Torgov reaction
-unsaturated, synth. by Torgov reaction 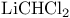 )
)  )
) 
 via enol ethers
via enol ethers 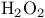 via
via 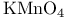


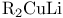 or RZnX
or RZnX 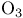
 - (lanosterol), biosynthesis, syntheses relating to
- (lanosterol), biosynthesis, syntheses relating to  , catalyst for aldol type condensation
, catalyst for aldol type condensation 
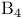 , synthesis
, synthesis  ), halogen-metal exchange with
), halogen-metal exchange with 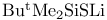 from
from  ), lithiation of allylic CH groups
), lithiation of allylic CH groups 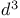 -synthon
-synthon 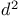 -synthon
-synthon  ), selective enolization with
), selective enolization with