|
|
 |
| Àâòîðèçàöèÿ |
|
|
 |
| Ïîèñê ïî óêàçàòåëÿì |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. — Organic Synthesis: Concepts, Methods, Starting materials |

Îáñóäèòå êíèãó íà
Íàøëè îïå÷àòêó?
Âûäåëèòå åå ìûøêîé è íàæìèòå Ctrl+Enter
|
Íàçâàíèå: Organic Synthesis: Concepts, Methods, Starting materials
Àâòîðû: Fuhrhop J., Penzlin G.
ßçûê: 
Ðóáðèêà: Õèìèÿ/
Ñòàòóñ ïðåäìåòíîãî óêàçàòåëÿ: Ãîòîâ óêàçàòåëü ñ íîìåðàìè ñòðàíèö
ed2k:
Èçäàíèå: second edition, rewise and enlarged
Ãîä èçäàíèÿ: 1994
Êîëè÷åñòâî ñòðàíèö: 432
Äîáàâëåíà â êàòàëîã: 29.10.2005
Îïåðàöèè: Ïîëîæèòü íà ïîëêó |
Ñêîïèðîâàòü ññûëêó äëÿ ôîðóìà | Ñêîïèðîâàòü ID
|
|
 |
| Ïðåäìåòíûé óêàçàòåëü |
Steroids 277—289
Steroids, 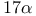 -ethynylation 62—63 278 -ethynylation 62—63 278
Steroids, 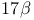 side-chain, construction 27 49 282—283 side-chain, construction 27 49 282—283
Steroids, 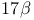 side-chain, oxidative degradation 118—119 283—286 side-chain, oxidative degradation 118—119 283—286
Steroids, 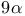 -fluorination 287 -fluorination 287
Steroids, 2- vs. 4-alkylation of 4- and 5-en-3-ones 25 156
Steroids, 21-hydroxylation 122
Steroids, conversions of natural steroids 283—289
Steroids, glycosylation of cardenolides 267
Steroids, pr. (table) 284
Steroids, redn. of the 8,9-double bond 101 104 278
Steroids, ring A, aromatization 287—288
Steroids, ring B, electrocyclic opening 289
Steroids, ring D, acid-catd. cyclization 91—92 279—280
Steroids, ring D, classic synthesis steps 49 54 55 59 82
Steroids, ring D, preformed cyclopentane units, introduction 71 73 280—281
Steroids, ring D, preformed cyclopentane units, synthesis 81 280—281
Steroids, Torgov’s synthesis 62—63 71 278
Steroids, total synthesis by acid-catalysed cyclization of a polyenynol 91—92 279—280
Steroids, total synthesis by acid-catalysed cyclization of ketone precursors 71 278—279
Steroids, total synthesis, intramol. Diels — Alder cyclizn. 280—281
Steroids, total synthesis, Robinson anellations 72—73
Stevens rearrangement 38 338
Stieglitz rearrangement see “sec. Amines N-halo-”
Stigmasta-5,22-dien-3-ol, 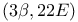 -, degradation of the -, degradation of the 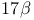 side-chain 285 side-chain 285
Stigmasta-5,22-dien-3-ol, 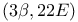 -, pr. 284 -, pr. 284
Stigmastan-3-ol, 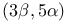 -, remote oxidation 286 -, remote oxidation 286
Stilbene diamine see “Stien”
Stilbenes, synthesis by Heck coupling 43
Stille coupling, aryl tridate + organylstannane 42
Stobbe condensation 58—59
Stork reagent 328—329
Strain, steric see “Steric strain”
Strecker’s synthesis of  -amino acids 50 301 -amino acids 50 301
Styrene see “Benzene ethenyl-”
Succinic acid see “Butanedioic acid”
Succinimide see “2
Sugars see “Monosaccharides” “Oligosaccharides”
Sugars, inexpensive derivs., pr. (table) 263—264
Sulfafurazole 307
Sulfamethoxazole 308
Sulfenic acids, esters, penam-cephem rearrangement via 315
Sulfenic acids, halides, penam-cephem rearrangement via 314
Sulfenic acids, halides, Reetz carbosulfenylation of alkenes 21
Sulfide contraction see “Sulfur extrusion”
Sulfides, diorganyl- see “Thioethers”
Sulfisoxazole 307
Sulfitolysis, oxidative 239—240
Sulfones, alkyl d-synthons 70
Sulfones, anions of 8 70
Sulfones, Julia — Lythgoe olefination with 34
Sulfones, reduction to thioethers 115
Sulfones, reductive desulfurization 70
Sulfones, synth. by oxn. of thioethers 80 216—217
Sulfonic acids, catalysts for cationic reactions, acetal hydrolysis 337
Sulfonic acids, catalysts for cationic reactions, acetalizations and transacetalizations 165—166 268 270 277 314 321—322
Sulfonic acids, catalysts for cationic reactions, acetalizations and transacetalizations, solid-phase (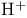 resins) 267 resins) 267
Sulfonic acids, catalysts for cationic reactions, acid-catalysed cyclizations 278 335—336
Sulfonic acids, catalysts for cationic reactions, aldol condensation (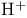 resin) 82 resin) 82
Sulfonic acids, catalysts for cationic reactions, esterifications and transesterifications 58 65 70 325 328
Sulfonic acids, catalysts for cationic reactions, esterifications and transesterifications, solid-phase (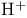 resin) 282 resin) 282
Sulfonic acids, esters, 1,2-cioi esters, oxiranes from 269
Sulfonic acids, esters, 1,2-cioi esters, pinacol rearr. of 32
Sulfonic acids, esters, 1-cyclopropylalkyl esters, 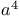 -synthons 14—15 17 70 76 -synthons 14—15 17 70 76
Sulfonic acids, esters, 1-decalyl esters, fragmentation 89—90
Sulfonic acids, esters, a-synthons 15—16 24 46—47 93 125 267 291
Sulfonic acids, esters, aryl esters, Stille coupling 42
Sulfonic acids, esters, oxime “esters”, Beckmann rearr. 136—137
Sulfonic acids, esters, trialkylsilyl esters, O-silylation with 321—322 325—326 328—329
Sulfonium salts, Hofmann elimn. of 38 338
Sulfonium ylides, prepn. and stability 7—8 45
Sulfonium ylides, reactions with carbonyl groups 45 79
Sulfonium ylides, reactions with electrophilic alkenes 75—76
Sulfonium ylides, reactions with imines 154
Sulfonium ylides, reactions with triorganylboranes 9
Sulfonium, dimethyl(methylthio)-, ![$\mathrm{[Me_{2}SSMe]CF_{3}SO_{3}}$](/math_tex/ed49d38a44fc8f7993137c34e56d069582.gif) for activation of thioglycosides 271 for activation of thioglycosides 271
Sulfonium, dimethyl-, 2-ethoxy-2-oxoethylide 8
Sulfonium, dimethyl-, methylide (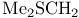 ), oxiranation of carbonyl compounds 45 ), oxiranation of carbonyl compounds 45
Sulfonium, dimethyl-, methylide (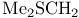 ), preparation 8 45 ), preparation 8 45
Sulfonium, dimethyl-, methylide, oxide see “Sulfoxonium”
Sulfonium, diphenyl-, cyclopropylide (Trost’s reagent), syntheses with 79 335—336
Sulfonium, trimethyl-, iodide (![$\mathrm{[Me_{3}S]I}$](/math_tex/dd96a673b64b25903a343225a04abc0382.gif) ), pr. 181 ), pr. 181
Sulfonium, trimethyl-, iodide (![$\mathrm{[Me_{3}S]I}$](/math_tex/dd96a673b64b25903a343225a04abc0382.gif) ), ylide from 8 45 ), ylide from 8 45
Sulfonium, trimethyl-, iodide, oxide see “Sulfoxonium”
Sulfoxide, dimethyl- see “Methane sulfinylbis[-”
Sulfoxide, methyl methylthiomethyl see “Methane (methylsulfinyl)(methylthio)-”
Sulfoxides,  -elimn. of sulfenic acids from 65 86 315 -elimn. of sulfenic acids from 65 86 315
Sulfoxides,  -oxo- see “Ketones -oxo- see “Ketones
Sulfoxides, anions of 8—9
Sulfoxides, chiral sulfur 8—9
Sulfoxides, Pummerer rearrangement 51 265
Sulfoxides, reduction to thioethers 115 229
Sulfoxonium, dimethyl-, methylide ( ), aziridination of imines 154 ), aziridination of imines 154
Sulfoxonium, dimethyl-, methylide ( ), cyclopropanation of 2-enones etc. 75—76 ), cyclopropanation of 2-enones etc. 75—76
Sulfoxonium, dimethyl-, methylide ( ), oxiranation of carbonyl compounds 45 ), oxiranation of carbonyl compounds 45
Sulfoxonium, dimethyl-, methylide ( ), preparation 8 45 ), preparation 8 45
Sulfoxonium, trimethyl-, iodide (![$\mathrm{[Me_{3}SO]I}$](/math_tex/fe44b8eaa0b13fec030a5d37fadcecfa82.gif) ), pr. 181 ), pr. 181
Sulfoxonium, trimethyl-, iodide (![$\mathrm{[Me_{3}SO]I}$](/math_tex/fe44b8eaa0b13fec030a5d37fadcecfa82.gif) ), ylide from 8 45 154 ), ylide from 8 45 154
Sulfur extrusion by Stevens rearr. 38 338
Sulfur extrusion by sulfone pyrolysis 80 153
Sulfur extrusion with phosphines or trialkyl phosphites 35 38—39 59—60 261 334
Sulfur ylides see “Sulfonium ylides”
Sulfur, (N-ethylethanalmnato)trifluoro- (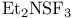 , DAST), OH/F exchange 269—270 111 , DAST), OH/F exchange 269—270 111
Sulfur, diphenylbis[2,2,2-trifluoro-1-phenyl-1-(trifluoromethyl)ethoxy]- (![$\mathrm{Ph_{2}S[OC(CF_{3})_{2}Ph]}$](/math_tex/56f15824fb5dccd54913458fc5c9a5c382.gif) , Martin’s reagent), dehydration with 282 , Martin’s reagent), dehydration with 282
Sulfurane see “Sulfonium” “Sulfur”
Sulfuryl chloride isocyanate (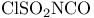 , CST), cycloaddition with 153 , CST), cycloaddition with 153
Supramolecular chemistry 350
Swern oxidation (ClCOCOCl/DMSO) 133—134 322—323 325—327
syncat = syn-catenoid, definition 360
Synthons for N-heterocycles 147—149 152—153
Synthons, definition 1—3 4 193—196
Synthons, examples see “Acceptor synthons” “Donos “Electrocyclizations” “Radical
Tachysterols = 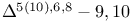 -seco-sterols 34 289 -seco-sterols 34 289
Taq DNA polymerase 227
Tartaric acid, derivs., enantioselective catalysts for aldol type addition 61
Tartaric acid, derivs., enantioselective catalysts for Sharpless epoxidation 124—127 265 321 322 324—325
Tartaric acid, derivs., optical resolution with 300 301 309 327
Tartaric acid, derivs., pr. 185
Tbeoc = (2,2,2-tribromoethoxy)carbonyl, protecting group 157—158 163
TBHP see “Hydroperoxide (1
Tce = 2,2,2-trichloroethyl, protecting group 165 216 218 220
Tceoc = (2,2,2-trichldroethoxy)carbonyl, protecting group 157—158 162—163
TCQ see “Cyclohexadienedione tetrachloro-”
Tebbe’s methylation reagent 110 272
Template cyclizations 248 257 260 262
Terbinafine 302—303
Terminal transferase 242 243
Terpenes, cyclic see “Steroids”
Terpenes, cyclic, hydration via boranes 130—131
Terpenes, cyclic, oxn. of allylic CH 120—121
Terpenes, cyclic, pr. 189—192
Terpenes, cyclic, syntheses, acid catd. cyclizn 90—91 93
Terpenes, cyclic, syntheses, Diels — Alder cyclization 81—82 85—86
Terpenes, cyclic, syntheses, olefination 31—33
Terpenes, cyclic, syntheses, pinacol coupling 53
Terpenes, cyclic, syntheses, Robinson anellation 71—73
Terpenes, open-chain see “Carotenoids” “Juvenile
Terpenes, open-chain, acid-catalysed cyclization of 90—91
Terpenes, open-chain, alkylation of, via thioetbers 26
Terpenes, open-chain, homologation with acetoacetic esters 24
Terpenes, open-chain, synthesis of, Julia’s method 69—70 77
Terpenes, open-chain, synthesis of, Reformatsky reaction 44—45
Terpenes, open-chain, synthesis of, sulfur extrusion methods 39
Terramycin 317
tert-Butanol see “2-Propanol 2-methyl-”
tert-Butyl hydroperoxide see “Hydroperoxide 1
tert. Amines, cleavage with BrCN 309
tert. Amines, elimination of 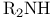 57 73 57 73
tert. Amines, N-oxides, deoxygenation 115
tert. Amines, N-oxides, oxidants in 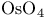 -catalysed oxn. 129 277 -catalysed oxn. 129 277
| tert. Amines, oxidation to enamines or iminium ions 116 120—121 138—139
tert. Amines, synthesis by redn. of carboxamides 98 111 247 291
tert. Amines, synthesis by redn. of iminium ions 293
Tetracarbonylferrate(2-) see “Ferrate(2-) ...”
Tetracyclines 316—318
Tetracyclo[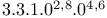 ]nonan-3-one 94 ]nonan-3-one 94
Tetrahedrane = ![$tricyclo[1.1.0.0^{2,4}]butane$](/math_tex/8cf7c735d9e4ca2ac0799fa0b699242082.gif) 330 330
Tetrahydropyranyl see “Protection”
Tetrapyrrole pigments 250—262
Tetratriacontanedioic acid 20
Tetrazole, phosphoramidite coupling catalyst 222
Thallium(1+), catalysts for displacement of tosylate 49 51
Thallium(1+), catalysts for oxn. of alkenes with 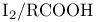 127 127
Thallium(3+) acetate ( , TTA), oxdiation of alkynes 117 132 , TTA), oxdiation of alkynes 117 132
Thallium(3+) acetate ( , TTA), oxidation of alkenes 117 127—128 , TTA), oxidation of alkenes 117 127—128
Thallium(3+) nitrate (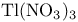 , TTN) or trifluoroacetate ( , TTN) or trifluoroacetate (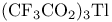 , TTFA), ox. cleavage of 1-alkynes with 117 132 , TTFA), ox. cleavage of 1-alkynes with 117 132
Thallium(3+) nitrate (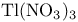 , TTN) or trifluoroacetate ( , TTN) or trifluoroacetate (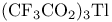 , TTFA), ox. rearr. with, of alkenes 117 129—130 , TTFA), ox. rearr. with, of alkenes 117 129—130
Thallium(3+) nitrate (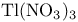 , TTN) or trifluoroacetate ( , TTN) or trifluoroacetate (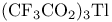 , TTFA), ox. rearr. with, of ketones 118 136 , TTFA), ox. rearr. with, of ketones 118 136
Thermodynamic control, acid-catd. cyclizn 90—91 93 168 279—280
Thermodynamic control, diaslereoselective lactonization 168 327—328
Thermodynamic control, enolate formn 11—12 24—25 55 58 63 93
Thermus aquaticus DNA polymerase 227
Thexylborane see “Borane (1
Thiiranes, reductive desulfurization, Barton olefinations 35
Thiiranes, reductive desulfurization, Eschenmoser’s sulfur extrusion 59—60 261
Thioacetals see “1 “1 “Monothioacetals” “1
Thioesters see “Carbothioic acids S-esters” “Sulfonothioic S-esters”
Thioethers,  -chlorination 314 -chlorination 314
Thioethers,  -elimination via sulfohium salts 38 338 -elimination via sulfohium salts 38 338
Thioethers, allylic, a-alkylation of anions of 26
Thioethers, allylic, rearrangements 39
Thioethers, fluorides from (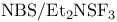 ) 269—270 ) 269—270
Thioethers, oxidative cleavage 314—315
Thioethers, rearrangements 38 39 338
Thioethers, reductive desulfurization with alkali metals 39 98 113—114
Thioethers, reductive desulfurization with hydrogen/Ni-B 26
Thioethers, reductive desulfurization with Raney 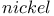 76 82 98 109 113—114 156 273 76 82 98 109 113—114 156 273
Thioethers, reductive desulfurization with triphenylstannane 110—111
Thioethers, S-alkylation 38 39 338
Thioethers, S-chlorination and cleavage 314—315
Thioethers, S-oxidation to sulfones 80 216 217
Thioethers, S-oxidation to sulfoxides 315
Thioethers, S-oxidation to sulfoxides for Pummerer rearr. 265
Thioethers, sulfur extrusion from 38—39 80 338
Thioethers, synthesis by carbosulfenylation of alkenes 21
Thioethers, synthesis from diorganyl disulfides 39
Thioethers, synthesis from thiol and enoic esters 269
Thioethers, synthesis from thiol and halide 80 273 333 356
Thioethers, vinylic, hydrolysis 83
Thioethers, vinylic, synthesis by Peterson olefination 33—34
Thioethers, vinylic, synthesis by Wittig olefination 32 83
Thioethers, vinylic, synthesis via  -(methylsulfinyl) ketones 51 -(methylsulfinyl) ketones 51
Thioglycosides, glycosylation with 271
Thioglycosides, synthesis 269
Thioketals see “1 “1 “Monothioacetals” “1
Thiolactams, S extrusion 59—60 261
Thiols, synthesis from haloalkanes 169 269
Thionation of lactones 110—111
Thiones, olefination of 35
Thionocarbonates see “Carbonothioates”
Thionolactones 110—111
Thionyl chloride, prepn. of acyl halides 143
Thionyl chloride, prepn. of carboxylic esters 347
Thionyl chloride, prepn. of haloalkanes from alcohols 32
Thiophenol see “Benzenethiol”
Thiotosylate see “Benzenesulfonothioic acid”
Thiourea, conversion of haloalkanes to thiols 169 269
Thiourea, O-O cleavage of peroxides 276—277
Thp = tetrahydropyranyl see “Protection”
threo, definition 62 360
threo-Selectivity see “erythro/threo”
Threonine, pr. 184
Threonine, protection 229 241
Tin organyls see “Stannane”
Tin(2+) chloride, activation of glycosyl fluorides with 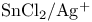 270 270
Tin(2+) chloride, reduction of nitroarenes 253
Tin(4+) chloride, Lewis acid catalyst, aldol type reactions 93
Tin(4+) chloride, Lewis acid catalyst, Friedel — Crafts alkylations 233 256—257
Titanium(0), McMurry coupling 41
Titanium(2+), pincaol coupling 53
Titanium(4+), chloride tris(2-propanolate), aldol addition catalyst 62
Titanium(4+), tetrachloride, Lewis acid catalyst, aldol type reactions 56 58
Titanium(4+), tetrachloride, Lewis acid catalyst, allylstannane addn. to aldehydes 66—67
Titanium(4+), tetrachloride, Lewis acid catalyst, carbosulfenylation of alkenes (Reetz) 21
Titanium(4+), tetrachloride, Lewis acid catalyst, Michael addn. of allylsilanes (Sakurai) 90
Titanium(4+), tetrachloride, Lewis acid catalyst, opening of cyclopropanone ketals 14—15 70
Titanium(4+), tetrakis(2-propanolate), catalyst, aldol type addition 62
Titanium(4+), tetrakis(2-propanolate), catalyst, Sharpless epoxn. 124—127 265 321—326
Titanium, 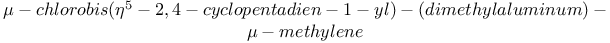 - (Tebbe’s reagent), methylenation with 110 272 - (Tebbe’s reagent), methylenation with 110 272
TMM = “trimethylenemethane” synthon 84
Tolbutamide 301
Toluene... see “Benzene... methyl-”
Toluquinone see “Cyclohexadienedione methyl-”
Torgov’s steroid synthesis, final steps 278
Torgov’s steroid synthesis, first step 62—63
Torgov’s steroid synthesis, second step 71
TosMIC = 1-[(isocyanomethyl)sulfonyl]-4-methylbenzene (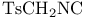 ) 49 51 ) 49 51
Tosylates see “Benzenesulfonic acid 4-methylesters”
Tosylhydrazine see “Benzenesulfonic acid 4-methyl- hydrazide”
TPP = 5,10,15,20-tetraphenylporphine 252
Tpte protecting group 216 217
Trajectories in reactions of nucleophiles 316
trans-Selectivity see “cis/trans”
Transcriptase, reverse 242
Transform, retro-synthetic 193—196
Triamterene 308
Triasteranone 94
Tricyclo[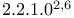 ]heptane, 1,7-dimethyl-7-(4-methyl-3-pentenyl)- ( ]heptane, 1,7-dimethyl-7-(4-methyl-3-pentenyl)- ( -santalene) 42 -santalene) 42
Tricyclo[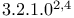 ]oct-6-ene, ]oct-6-ene, 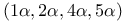 - 92 - 92
Tricyclo[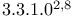 ]nona-3,6-dien-9-one 94 ]nona-3,6-dien-9-one 94
Tricyclo[ ]octa-3,7-dienes, syn- 330 332 ]octa-3,7-dienes, syn- 330 332
Tricyclo[ ]decan-3-one 3 ]decan-3-one 3
Tricyclo[ ]decan-3-one, 6-methyl-, retro-synthesis 211—212 ]decan-3-one, 6-methyl-, retro-synthesis 211—212
Tricyclo[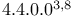 ]decan-4-one, 8-(acetyloxy)-, synthesis 93 ]decan-4-one, 8-(acetyloxy)-, synthesis 93
Tricyclo[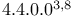 ]decan-4-one, synthesis 93 ]decan-4-one, synthesis 93
Triflates see “Methanesulfonic acid trffluoro- esters”
Trifunctional compounds, pr. 182—185 189 191 192
Trifunctional compounds, retro-synthetic analysis 202 207
Triiluoroacetyl protecting group 162 267
Trimesic acid, hydrogenation 347
Trimethylsilyl see “Protection”
Triorganylboranes see “Boranes”
Triprolidine 304
Triptycene = 9,10-dihydro-9,10-[1',2']benzenoanthracene, synthesis 93
Triquinacene = 2a,4a,6a,6b-tetrahydrocyclopenta[cd]pentalene, cyclodimerization attempts 335
Triquinacene = 2a,4a,6a,6b-tetrahydrocyclopenta[cd]pentalene, synthesis steps 141 143 165 333
Triton B see “Benzenemethanaminium ...”
Trityl anion see “Lithium (triphenylmethyl)-” “Potassium (triphenylmethyl)-”
Trityl cation see “Methylium triphenyl-”
Trityl protecting group see “Protection”
Tritylium cation see “Methylium triphenyl-”
Troponimine, 2-amino-, “chiramt” derivative, enantioselective organocuprate addn 20—21
Trost’s reagent, diphenylsulfonium cyclopropylidc 79 335—336
Tryptamine, alkaloids from 291—293
Tryptamine, pr. 290
Tsuchihashi — Pummerer rearrangement 265
TTFA see “Thallium(3+) trifluoroacetate”
TTN see “2'-Deoxynucleoside 5'-triphosphates”
Twistane derivatives 93
Two-directional chain synthesis 327—328
Tyrosine, protection 229
Ullmann reaction 294—295
Umpolung, definition 1 17
Umpolung, examples 17—19
Umpolung, examples, 1-alkyne to 1-halo-1-alkyne 40
Umpolung, examples, alkene to oxirane 211
Umpolung, examples, nitroalkane to ketone 16 65—66
Undecanal, Peterson olefination of 34
Undecanoic acid, 11—halo—, alkyl coupling with 20
Undecanoic acid, 11—halo—, pr. 180
|
|
 |
| Ðåêëàìà |
 |
|
|
 |
|
Î ïðîåêòå
|
|
Î ïðîåêòå



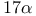 -ethynylation
-ethynylation 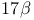 side-chain, construction
side-chain, construction 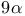 -fluorination
-fluorination 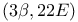 -, degradation of the
-, degradation of the 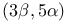 -, remote oxidation
-, remote oxidation  -amino acids
-amino acids 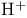 resins)
resins) 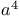 -synthons
-synthons ![$\mathrm{[Me_{2}SSMe]CF_{3}SO_{3}}$](/math_tex/ed49d38a44fc8f7993137c34e56d069582.gif) for activation of thioglycosides
for activation of thioglycosides 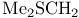 ), oxiranation of carbonyl compounds
), oxiranation of carbonyl compounds ![$\mathrm{[Me_{3}S]I}$](/math_tex/dd96a673b64b25903a343225a04abc0382.gif) ), pr.
), pr.  -elimn. of sulfenic acids from
-elimn. of sulfenic acids from  ), aziridination of imines
), aziridination of imines ![$\mathrm{[Me_{3}SO]I}$](/math_tex/fe44b8eaa0b13fec030a5d37fadcecfa82.gif) ), pr.
), pr. 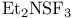 , DAST), OH/F exchange
, DAST), OH/F exchange ![$\mathrm{Ph_{2}S[OC(CF_{3})_{2}Ph]}$](/math_tex/56f15824fb5dccd54913458fc5c9a5c382.gif) , Martin’s reagent), dehydration with
, Martin’s reagent), dehydration with 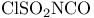 , CST), cycloaddition with
, CST), cycloaddition with 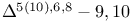 -seco-sterols
-seco-sterols 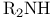
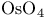 -catalysed oxn.
-catalysed oxn. 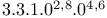 ]nonan-3-one
]nonan-3-one ![$tricyclo[1.1.0.0^{2,4}]butane$](/math_tex/8cf7c735d9e4ca2ac0799fa0b699242082.gif)
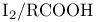
 , TTA), oxdiation of alkynes
, TTA), oxdiation of alkynes 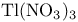 , TTN) or trifluoroacetate (
, TTN) or trifluoroacetate (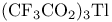 , TTFA), ox. cleavage of 1-alkynes with
, TTFA), ox. cleavage of 1-alkynes with 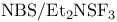 )
) 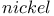
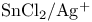
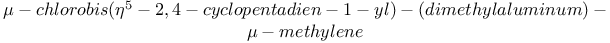 - (Tebbe’s reagent), methylenation with
- (Tebbe’s reagent), methylenation with 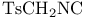 )
) 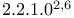 ]heptane, 1,7-dimethyl-7-(4-methyl-3-pentenyl)- (
]heptane, 1,7-dimethyl-7-(4-methyl-3-pentenyl)- (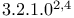 ]oct-6-ene,
]oct-6-ene, 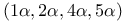 -
- 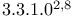 ]nona-3,6-dien-9-one
]nona-3,6-dien-9-one  ]octa-3,7-dienes, syn-
]octa-3,7-dienes, syn-  ]decan-3-one
]decan-3-one 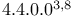 ]decan-4-one, 8-(acetyloxy)-, synthesis
]decan-4-one, 8-(acetyloxy)-, synthesis