|
|
 |
| Àâòîðèçàöèÿ |
|
|
 |
| Ïîèñê ïî óêàçàòåëÿì |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. — Organic Synthesis: Concepts, Methods, Starting materials |

Îáñóäèòå êíèãó íà
Íàøëè îïå÷àòêó?
Âûäåëèòå åå ìûøêîé è íàæìèòå Ctrl+Enter
|
Íàçâàíèå: Organic Synthesis: Concepts, Methods, Starting materials
Àâòîðû: Fuhrhop J., Penzlin G.
ßçûê: 
Ðóáðèêà: Õèìèÿ/
Ñòàòóñ ïðåäìåòíîãî óêàçàòåëÿ: Ãîòîâ óêàçàòåëü ñ íîìåðàìè ñòðàíèö
ed2k:
Èçäàíèå: second edition, rewise and enlarged
Ãîä èçäàíèÿ: 1994
Êîëè÷åñòâî ñòðàíèö: 432
Äîáàâëåíà â êàòàëîã: 29.10.2005
Îïåðàöèè: Ïîëîæèòü íà ïîëêó |
Ñêîïèðîâàòü ññûëêó äëÿ ôîðóìà | Ñêîïèðîâàòü ID
|
|
 |
| Ïðåäìåòíûé óêàçàòåëü |
Magnesium, bromoethenyl-, Michael type ethenylation with Cu(1+) 281
Magnesium, bromoethyl-, metalation of 1-alkynes 5 155
Magnesium, chloro[(trimethylsilyl)methyl]-, Peterson olefination with 33
Magnesium, chloro[(trimethylsilyl)methyl]-, preparation 6—7
Magnesium, iodomethyl-, selective methylation 318
Magnesium, metalation of organyl halides 5 6—7 17 20 46
Malabaricanediol,  - and - and  -18,19-epi- 91 -18,19-epi- 91
Maleic anhydride see “2
Malic acid see “Butanedioic acid 2-hydroxy-”
Malonic acid see “Propanediol acid”
Malononitrile see “Propanedinitrile”
Manganese(4+) oxide, oxidation with, of aldehydes via cyanohydrins 134—135
Manganese(4+) oxide, oxidation with, of allylic alcohols 133—135
Mannich bases see “Ketones
Mannich reactions 57 291—292 300 304 310 317—318
Mannich reagents see “Iminium ions”
Marker degradation 283/285
Martin’s sulfurane reagent, dehydration by 282
Masamune reaction, aldol addition of chiral  -silyloxy ketones 62 -silyloxy ketones 62
McMurry olefination 41
Mcpba see “Benzenecarboperoxoic acid 3-chloro-”
Meclizine = meclozine,  - 304 - 304
Meerwein — Ponndorf redn. see “Oppenauer oxn.”
Meerwein — Ponndorf redn. with  302 302
Meerwein — Ponndorf redn., ax/eq equilibration of cycloalkanols 288
Meerwein’s reagents see “Methylium dimethoxy-” “Oxonium triethyl-” “Oxonium trimethyl-”
Membranes, bilayer (BLM), from lipids or synthetic amphiphiles 350—351
Membranes, bilayer (BLM), permeability enhancement,  243—244 243—244
Membranes, monolayer, from bola amphiphiles 351
Menthol, (-)-(1R)-, (+)-(1S)- and  -, asym. aldol type addition with esters of 59 -, asym. aldol type addition with esters of 59
Menthol, (-)-(1R)-, (+)-(1S)- and  -, pr. 189 -, pr. 189
Meprobamate 302
Mercury(2+) acetate, hydration of alkenes 283
Mercury(2+) acetate, oxn. of allylic or benzylic CH 116 120—121
Mercury(2+) acetate, oxn. of tert. amines 116 120—121 138—139
Mercury(2+), catalyst for alcohol addition to alkynes 64—65 325 327—328
Mercury(2+), catalyst for glycosylations (Koenigs — Knorr) 267
Mercury(2+), catalyst for hydration of alkynes 52 57 117
Mercury(2+), catalyst for hydrolysis of 1-alkenyl halides 63
Mercury(2+), catalyst for hydrolysis of dithioacetal derivs. 22 66 156 267
Mercury(2+), catalyst for hydrolysis of monothioacetal derivs. 76 229
Mercury(2+), catalyst for hydrolysis of thiocarbamates 229
Mercury(2+), catalyst for hydrolysis of trityl thioethers 229
Merrifield’s peptide synthesis 232—237
Mesembrine,  - 298 - 298
Mesoionic intermediates in cycloadditions 153
Mesoionic intermediates in electrocyclizations 262 295—296
Mesoporphyrin 256
Messenger-RNA (mRNA) for gene cloning 242
Mesylates see “Methanesulfonic acid esters”
meta-Cyclophanes 38 338
Metal organyls see “Organometallic compounds”
MethanaL see “Formaldehyde”
Methanamine, 1,1-dimethoxy-N,N-dimethyl-, formylation with 82
Methanamine, N,N-dimethyl-, N-oxide, oxn. of 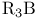 37 37
Methane, (methylsullmylXmethylthio)- (MMTS), synthesis of ketones 66 205
Methane, chloromethoxy- ( ), Friedel — Crafts chloromethylation with 233 ), Friedel — Crafts chloromethylation with 233
Methane, chloromethoxy- ( ), methoxymethylation with 210 ), methoxymethylation with 210
Methane, chloromethoxy- ( ), O-methoxymethyl (MOM) protection 318 ), O-methoxymethyl (MOM) protection 318
Methane, diazo- (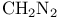 ), carbenoid reactions of 74—75 166 334 ), carbenoid reactions of 74—75 166 334
Methane, diazo- (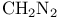 ), cyclopropanone synthesis with 75 ), cyclopropanone synthesis with 75
Methane, diazo- (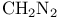 ), prepn. of methyl esters with 82 325 ), prepn. of methyl esters with 82 325
Methane, diazo- (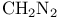 ), ring expansion of cyclic ketones with 83 ), ring expansion of cyclic ketones with 83
Methane, dichloromethoxy- ( ), methoxycarbene from 74 ), methoxycarbene from 74
Methane, dichloromethoxy- ( ), pr. 179 ), pr. 179
Methane, diiodo-, carbene synthon 74—75 83
Methane, iodo-,  -methyl lactones 321 322 328 -methyl lactones 321 322 328
Methane, iodo-, S-methylation of thiols 110—111
Methane, sulfinylbis[- (dimethyl sulfoxide, DMSO), acidity 10
Methane, sulfinylbis[- (dimethyl sulfoxide, DMSO), dehydration of alcohols with 140
Methane, sulfinylbis[- (dimethyl sulfoxide, DMSO), Moffatt — Pfitzner and Swern oxn. of alcohols 133—134 322—323 325—327
Methane, sulfinylbis[-, ion(1-) (”dimsyl anion”),  -synthon 6 8 50—51 65 -synthon 6 8 50—51 65
Methane, sulfinylbis[-, ion(1-) (”dimsyl anion”), deprotonation with, of carbonyl compounds 10 83
Methane, sulfinylbis[-, ion(1-) (”dimsyl anion”), deprotonation with, of phosphonic diesters 30
Methane, sulfinylbis[-, ion(1-) (”dimsyl anion”), deprotonation with, of sulfonium salts 8
Methane, tetrahalo-, halides from prim, alcohols,  (X=Cl, Br, I) 266 (X=Cl, Br, I) 266
Methane, tetrahalo-, halides from prim, alcohols, oxn. of phosphonic diesters,  343 343
Methane, tribromo-,  from 75 from 75
Methane, trichloro-, lithiated 51—52
Methane, trimethoxy see “Orthoformic acid esters”
Methane, trimethylene- = 2-methylene-1,3-propanediyl, e-synthons for [3+2]cycloadditions 84
Methanediamine, 1-(1,1-dimethylethoxy)-N,N,N',N'-tetramethyl-, vinylogous amides from 79
Methanesulfenyl bromide (MeSBr), activation of thioglycosides 271
Methanesulfonic acid see “Sulfonic acids”
Methanesulfonic acid, 1-cyclopropylalkyl esters, 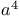 -synthons 15 17 70 76 -synthons 15 17 70 76
Methanesulfonic acid, 1-decalyl esters, fragmentation of 89—90
Methanesulfonic acid, alkyl esters (mesylates), a-synthons 15—17
Methanesulfonic acid, alkyl esters (mesylates), alkyl azides from 267
Methanesulfonic acid, trifluoro- (triflic acid), (1,1-dimethylethyl)dimethylsilyl ester, O-silylations with 321 322 325 328
Methanesulfonic acid, trifluoro- (triflic acid), anhydride with dibutylborinic acid (dibutylboryl triflate,  ) etc., boron enolates from 12—13 61—62 ) etc., boron enolates from 12—13 61—62
Methanesulfonic acid, trifluoro- (triflic acid), anhydride, triflate esters from 295
Methanesulfonic acid, trifluoro- (triflic acid), aryl esters (aryl triflates), Stille coupling with organylstannanes 42 295
Methanesulfonic acid, trifluoro- (triflic acid), catalyst for skeletal rearr. 335—336
Methanesulfonic acid, trifluoro- (triflic acid), methyl ester (methyl triflate,  ), S-methylation of dimethyl disulfide 271 ), S-methylation of dimethyl disulfide 271
Methanesulfonic acid, trifluoro- (triflic acid), trimethylsilyl ester ( ), activation of glycosyl imidates 270—271 ), activation of glycosyl imidates 270—271
Methanesulfonyl chloride (mesyl chloride, MsCl), prepn. of mesylate esters 70 76 267 283
Methanesullinic acid, hydroxy-, monosodium salt (SFS), redn. of thiohemiacetals with 51
Methanoic acid see “Formic acid”
Methanol, ion(1-), deblocking of O-acyl derivs. 157—158 160 271
Methanol, ion(1-), deprotonation with, of 1,3-diones 72—73
Methanol, ion(1-), deprotonation with, of ketones 84
Methanol, ion(1-), deprotonation with, of phosphonium salts 31
Methanol, ion(1-), desilylation of  -silyl ketones with 73 -silyl ketones with 73
Methanol, ion(1-), rearrangements of  -halo ketones 84 -halo ketones 84
Methanol, ion(1-), rearrangements of  -halo triorganylboranes 37—38 -halo triorganylboranes 37—38
Methanone, diphenyl-, aldol addn. with 56—57
Methionine, pr. 185
Methionine, protection 229
Methyldopa, (-)-L- 301
Methylenes see “Carbenes”
Methylidynation, 1-alkynes from aldehydes 325
Methylium, dimethoxy-, tetrafluoroborate (![$\mathrm{[(MeO)_{2}CH]BF_{4}}$](/math_tex/d2170cb3c5bb2763d5ceee530096480682.gif) ), formyl ), formyl 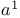 -synthon 58—59 -synthon 58—59
Methylium, dimethoxy-, tetrafluoroborate (![$\mathrm{[(MeO)_{2}CH]BF_{4}}$](/math_tex/d2170cb3c5bb2763d5ceee530096480682.gif) ), S-methylation of thioethers 38 ), S-methylation of thioethers 38
Methylium, triphenyl- (![$\mathrm{[Ph_{3}C]PF_{6}}$](/math_tex/5d60b520dbe69e227788f2cfe397410882.gif) or or ![$\mathrm{[Ph_{3}C]BF_{4}}$](/math_tex/ed35c69530c6fce8fa8b5ed9578f9f8982.gif) ), dehydrogenation with 44 249 334 ), dehydrogenation with 44 249 334
Methylphenidate,  - 303 - 303
Metronidazole 306
Micellar fibers of surfactants 352—353
Micelles, globular (= spherical) 353
Micelles, polymeric (= covalent), dendritic 354—356
Michael-type additions, 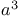 + alkyl d 20—21 + alkyl d 20—21
Michael-type additions,  274—275 291 316 351 354 274—275 291 316 351 354
Michael-type additions,  65—66 75—76 205 65—66 75—76 205
Michael-type additions,  71—74 205 318 339 71—74 205 318 339
Michael-type additions,  , reversibility 72 , reversibility 72
Michael-type additions,  (Sakurai reaction) 90 (Sakurai reaction) 90
Microbiological methods, literature 118
Microbiological methods, norgestrel synthesis 278
Mimoun’s complex see “Molybdenum ...”
Mismatch oligonucleotide primer 245—246
Mitsunobu type reactions 160—161
MMTS see “Methane (methylsulfinylXmethylthio)-”
Moffatt — Pfitzner or Swern oxidation of alcohols 133—134 323 325—327
Moffatt’s reagent 160 327—328
Molybdenum, (hexamethylphosphoric triamide-O)oxodiperoxy(pyridine)- (Mimoun’s complex, ![$\mathrm{[MoO(O_{2})_{2}(Hmpta)(Py)]}$](/math_tex/f4f276d80c9b6bc079532f2df922b95782.gif) ), ),  -hydroxylation of enolates 121—122 -hydroxylation of enolates 121—122
Molybdenum, (hexamethylphosphoric triamide-O)oxodiperoxy(pyridine)- (Mimoun’s complex, ![$\mathrm{[MoO(O_{2})_{2}(Hmpta)(Py)]}$](/math_tex/f4f276d80c9b6bc079532f2df922b95782.gif) ), ox. cleavage of dialkylborinic esters 60—61 ), ox. cleavage of dialkylborinic esters 60—61
Monocyclic compounds 3 (see also “Carbocycles” “Heterocycles”)
Monodisperse dendritic polymers 355
Monosaccharides, 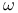 -deoxy- -deoxy-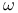 -halo-, synthesis 266 269—270 -halo-, synthesis 266 269—270
Monosaccharides, 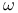 -deoxy-, by redn. of tosylates 114 -deoxy-, by redn. of tosylates 114
Monosaccharides, glycosyl halides 266 268—272
Monosaccharides, glycosyl imidates 270—271
Monosaccharides, homologization with 1,3-dithiane 267
Monosaccharides, homologization with cyanide/DIBAL 50
Monosaccharides, homologization, stereoselective (Wittig/Sharpless) 264—265
Monosaccharides, protection and activation 157—161 266—273
Monosaccharides, table, pr. 263—264
Monosaccharides, thioglycosides 269 271
Monosaccharides, total synthesis 264—265
Monothioacetals see “1
Monothioacetals, activation for substitution of sulfur 271
| Monothioacetals, cyclic, prepn. from thionolactones 110—111
Monothioacetals, cyclic, reductive desulfurization 110—111
Monothioacetals, elimination of thiols 265
Monothioacetals, reductive desulfurization 273
Monothioacetals, S-alkyl O-trialkylsilyi, non-enolizable 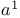 -synthons 15—16 -synthons 15—16
Monothioacetals, synthesis by Pummerer rearr. 51 265
Morphinane alkaloids by phenol coupling 294
Morpholine, enamines from 13—14 88
MPM = (4-methoxyphenyl)methyl, O-protective group 157 326—329
mRNA (messenger-RNA) for gene cloning 242
mRNA = messenger RNA, gene cloning 242
Mukaiyama aldol type reactions 58 62
Mukaiyama’s reagent 328—329
Mutagenesis, insertion of DNA 243—245
Mutagenesis, single-site mutations 245—246 341—343
myo-Inositol, selective glycosylation 270
N,N'-Dicyclohexylcarbodiimide see “Carbodiimide”
N-, P- and S-Oxides, deoxygenation 115
N-Bromoacetamide see “Acetamide N-bromo-”
N-Bromosuccinimide (= NBS) see “2 1-bromo-”
N-Heteroarenes, reduction of 112—113 212 303 309
N-Heteroarenes, syntheses of 146—153
N-Heteroarenes, syntheses of, by oxn. of heterocycles 120—121 148 249
N-Heteroarenes, syntheses of, drugs 307—310
N-Heteroarenes, syntheses of, indoles 151—152 296 307
N-Heteroarenes, syntheses of, tautomerism 148
N-Heterocycles 146—154 (see also “Lactams”)
N-Heterocycles by redn. of lactams 111—112 247 291
N-Heterocycles, 5-membered, syntheses 148—153
N-Heterocycles, 5-membered, syntheses, ene reactions 297—298
N-Heterocycles, 5-membered, syntheses, Fischer’s indole synth. 151—152 296 307
N-Heterocycles, 5-membered, syntheses, intramol. reactions 34—35 291 305—309
N-Heterocycles, 5-membered, syntheses, Knorr’s pyrrole synthesis 150—151
N-Heterocycles, 5-membered, syntheses, sigmatropic rearr. 298—299
N-Heterocycles, 5-membered, syntheses, Wittig cyclization 32
N-Heterocycles, 5-membered, syntheses, [2+3]cycloadditions 35 49 51 152—153
N-Heterocycles, 6-membered, syntheses 148—149
N-Heterocycles, 6-membered, syntheses, electrocyclization 295—296
N-Heterocycles, 6-membered, syntheses, hetero[2+4]cycloaddn 153—154 297
N-Heterocycles, 6-membered, syntheses, intramol. 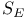 of arenes 291—293 of arenes 291—293
N-Heterocycles, 6-membered, syntheses, intramol. 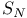 with nitrogen nucleophiles 291 294 304 306—308 310 with nitrogen nucleophiles 291 294 304 306—308 310
N-Heterocycles, 6-membered, syntheses, redn. of pyridines 112—113 303 309
N-Heterocycles, 7-membered, by intramol. 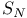 304 307 304 307
Naftifine 302
Naphthalene, 1,4,5,8-tetrahydro-, prepn. 104
Naphthalene, 1,4,5,8-tetrahydro-, selective epoxidation 124
Naphthalene, 4a,8a-dihydro-, cis-, photoisomers 332
Naphthalene, Birch redn. of 104
Naphthalene, naphthalene/K or Li, deprotonation with 52
Naphthalene, naphthalene/Na, red. deblocking with 220
NBA see “Acetamide N-bromo-”
NBS see “2 1-bromo-”
NCS see “2 1-chloro-”
Nef reaction 16 65—66
Neighboring group participation in reactions of 2-O-acylglycosyl compounds 270—272
Nickel(0),  -, allyl coupling 42 -, allyl coupling 42
Nickel(0),  -, trapping, with 1,2-propadiene 41 -, trapping, with 1,2-propadiene 41
Nickel(0),  -, trapping, with isocyanides 41 -, trapping, with isocyanides 41
Nickel(0), ![$di-mu-bromobis[(1,2,3-\eta)-3-methyl-2-butenyl]di$](/math_tex/6dee1be167449f1a587155e3a5f3dfa882.gif) -, terpene synthesis with 42 -, terpene synthesis with 42
Nickel(0), butadiene cyclooligomerizn. with 41
Nickel(0), tetracarbonyl-, allyl coupling with 42
Nickel(2+), catalyst for 2,3-reduction of 2-enones with  322—323 322—323
Nickel-aluminum alloy, activated see “Raney nickel”
Nitrenes = imidogens by oxidation of amines with Pb(4+) 212—213
Nitrenes = imidogens from azides 147—148 154
Nitric acid,  -nitro protection of arginine 229 -nitro protection of arginine 229
Nitrile N-oxides 153
Nitrile N-ylides = N-alkylidyne aminium inner salts 153
Nitriles to aldehydes 50 112 298
Nitriles to aldehydes via carboximidic esters 22—23 298
Nitriles to aldehydes via carboximidic esters to amines 50 98
Nitriles to aldehydes,  -spiroanellation 298 300 -spiroanellation 298 300
Nitriles to aldehydes, reductive cleavage (decyanidation) 280
Nitriles to aldehydes, synthesis from ketones with TosMIC 49
Nitriles,  -alkylation 200 298 300 302—304 -alkylation 200 298 300 302—304
Nitriles,  -hydroxy- (cyanohydrins) 18 50 -hydroxy- (cyanohydrins) 18 50
Nitriles, addn. of carbanions to 201 299
Nitriles, addn. of enolate type anions to 58—59
Nitriles, addn. of N-nucleophiles to 305 308
Nitriles, conversion to carboximidic esters 22—23 298
Nitriles, conversion to carboxylic esters 300 304
Nitriles, conversion to cyclic amidines 305
Nitriles, Diels — Alder addition to 154
Nitriles, heterocycles from 298—299 307—308
Nitriles, hydrolysis to amides 66 302 303 304
Nitroalkanes,  -synthons 6 65—66 301 302 -synthons 6 65—66 301 302
Nitroalkanes, carbon acidity 6
Nitroalkanes, conversion to carbonyl compds. 16 65—66
Nitroalkanes, reduction to amines 66 98 112 297 302
Nitroalkenes, 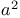 -synthons 16 -synthons 16
Nitroalkenes, diene addition to 297
Nitroalkenes, redn. to ketones 301
Nitroalkenes, synthesis 301
Nitroarenes, reduction to arenamines 253
Nitroarenes, reductive N,N-coupling 305
Nitrofurantoin 308
Nitrones = N-alkylidene amine N-oxides 153
Nitrosyl chloride, diazotization with 312—313
Nitrosyl chloride, nitrite esters from 286
Nitrosyl chloride, nitrosation of enol ethers 268
Nocardicinic acid, 3-amino- (ANA), 1,1-dimethylethyl ester, synthesis 161
Nonanedioic acid, 5-hydroxy-2,4,6,8-tetramethyl-, diastereoselective lactonization 168 327—328
Norbornane etc. see “Bicyclo[2.2.1]heptane” etc.
Norgestrel,  -, total synth. 278 -, total synth. 278
Norphedrine = (R*,S*)- -(1-aminoethyl)benzenemethanol, -(1-aminoethyl)benzenemethanol,  -, chiral auxiliary 61 -, chiral auxiliary 61
Norphedrine = (R*,S*)- -(1-aminoethyl)benzenemethanol, -(1-aminoethyl)benzenemethanol,  , synthesis 302 , synthesis 302
Noyori hydrogenation 102—103 325—326
Nucleophiles see “Donor synthons”
Nucleotides, oligonucleotide syntheses 215—224 341—343
Nucleotides, protection 157—160 166—167 216—224
Octane, 2-iodo-, (R)-, stereosel. acylation 22
OEP see “Porphine Octaethyl-”
Olefination see “Alkenes”
Olefins see “Alkenes”
Oleic acid see “9-Octadecenoic acid (Z)-”
Oligocyclic compounds 90—94 329—339 356—357
Oligocyclic compounds, bridged 92—94 329—338
Oligocyclic compounds, bridged, special syntheses, alkaloids 291 293—294 296—298
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, alkene + amine + 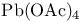 212—213 212—213
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, alkene + amine + 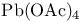 , intramolecular cyclopropanation 76 94 , intramolecular cyclopropanation 76 94
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, alkene + amine + 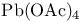 , oxidative phenol coupling 293—294 , oxidative phenol coupling 293—294
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, alkene + amine + 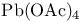 , photocyclizations 329—333 , photocyclizations 329—333
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, alkene + amine + 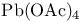 , pinacol coupling 53 , pinacol coupling 53
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, alkene + amine + 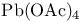 , polar cyclizations 93 211—212 291 309 330 334 , polar cyclizations 93 211—212 291 309 330 334
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, porphyrins, bridged 253
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, rearrangement, light-induced 329—333 337—338
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, rearrangement, metal-catd. or thermal 79—80 332—336
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, rearrangement, sulfur extrusion 38—39 338
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, rearrangement, tetrapyrrole pigments 250—262
Oligocyclic compounds, bridged, special syntheses, intramolecular aziridination, rearrangement, Wittig reactions 333—334
Oligocyclic compounds, bridged, special syntheses, [2+2]cycloadditions 78 276 297—298 333 337
Oligocyclic compounds, bridged, special syntheses, [2+4]cvcloadditions 78 85—86 92—93 209—210 212 297 331 333—337
Oligocyclic compounds, fused, fragmentation 89—90 141—142
Oligocyclic compounds, fused, special syntheses, 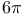 -electrocyclizations 38 295—296 338 -electrocyclizations 38 295—296 338
Oligocyclic compounds, fused, special syntheses, alkaloids 289—298
Oligocyclic compounds, fused, special syntheses, carbonylation of cyclic boranes 48
Oligocyclic compounds, fused, special syntheses, cationic 1, 5-diene cyclizations 90—92 279—280
Oligocyclic compounds, fused, special syntheses, cyclopentanellations 58—59 81
Oligocyclic compounds, fused, special syntheses, Diels — Alder anellations 78 80—82 84—86 280—281 297
Oligocyclic compounds, fused, special syntheses, drugs 307—308 310
Oligocyclic compounds, fused, special syntheses, ene reactions 297—298
Oligocyclic compounds, fused, special syntheses, indoles 151—152 296 307
Oligocyclic compounds, fused, special syntheses, Robinson anellations 71—73 298
Oligocyclic compounds, fused, special syntheses, tetracyclines 318
Oligocyclic compounds, fused, special syntheses, Torgov cyclization 278—279
Oligocyclic compounds, fused, special syntheses, [2+2+2]cyclotrimerization of alkynes 80 281 330
Oligocyclic compounds, fused, special syntheses, [2+2]cydoaddition 78
Oligocyclic compounds, retro-synthetic analysis 3 208—209 210—213
Oligocyclic compounds, spirocyclic see “Spiroanellation”
Oligocyclic hydrocarbon derivs., pr. 192
|
|
 |
| Ðåêëàìà |
 |
|
|
 |
|
Î ïðîåêòå
|
|
Î ïðîåêòå



 - and
- and  -silyloxy ketones
-silyloxy ketones 

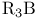
 ), Friedel — Crafts chloromethylation with
), Friedel — Crafts chloromethylation with 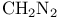 ), carbenoid reactions of
), carbenoid reactions of  ), methoxycarbene from
), methoxycarbene from  -synthon
-synthon  (X=Cl, Br, I)
(X=Cl, Br, I) 
 from
from 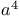 -synthons
-synthons  ) etc., boron enolates from
) etc., boron enolates from  ), S-methylation of dimethyl disulfide
), S-methylation of dimethyl disulfide  ), activation of glycosyl imidates
), activation of glycosyl imidates ![$\mathrm{[(MeO)_{2}CH]BF_{4}}$](/math_tex/d2170cb3c5bb2763d5ceee530096480682.gif) ), formyl
), formyl 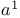 -synthon
-synthon ![$\mathrm{[Ph_{3}C]PF_{6}}$](/math_tex/5d60b520dbe69e227788f2cfe397410882.gif) or
or ![$\mathrm{[Ph_{3}C]BF_{4}}$](/math_tex/ed35c69530c6fce8fa8b5ed9578f9f8982.gif) ), dehydrogenation with
), dehydrogenation with 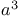 + alkyl d
+ alkyl d 


 (Sakurai reaction)
(Sakurai reaction) ![$\mathrm{[MoO(O_{2})_{2}(Hmpta)(Py)]}$](/math_tex/f4f276d80c9b6bc079532f2df922b95782.gif) ),
), 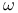 -deoxy-
-deoxy-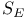 of arenes
of arenes 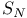 with nitrogen nucleophiles
with nitrogen nucleophiles  -, allyl coupling
-, allyl coupling ![$di-mu-bromobis[(1,2,3-\eta)-3-methyl-2-butenyl]di$](/math_tex/6dee1be167449f1a587155e3a5f3dfa882.gif) -, terpene synthesis with
-, terpene synthesis with 
 -nitro protection of arginine
-nitro protection of arginine 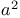 -synthons
-synthons  -, chiral auxiliary
-, chiral auxiliary 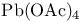
 -electrocyclizations
-electrocyclizations