|
|
 |
| Àâòîðèçàöèÿ |
|
|
 |
| Ïîèñê ïî óêàçàòåëÿì |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. — Organic Synthesis: Concepts, Methods, Starting materials |

Îáñóäèòå êíèãó íà
Íàøëè îïå÷àòêó?
Âûäåëèòå åå ìûøêîé è íàæìèòå Ctrl+Enter
|
Íàçâàíèå: Organic Synthesis: Concepts, Methods, Starting materials
Àâòîðû: Fuhrhop J., Penzlin G.
ßçûê: 
Ðóáðèêà: Õèìèÿ/
Ñòàòóñ ïðåäìåòíîãî óêàçàòåëÿ: Ãîòîâ óêàçàòåëü ñ íîìåðàìè ñòðàíèö
ed2k:
Èçäàíèå: second edition, rewise and enlarged
Ãîä èçäàíèÿ: 1994
Êîëè÷åñòâî ñòðàíèö: 432
Äîáàâëåíà â êàòàëîã: 29.10.2005
Îïåðàöèè: Ïîëîæèòü íà ïîëêó |
Ñêîïèðîâàòü ññûëêó äëÿ ôîðóìà | Ñêîïèðîâàòü ID
|
|
 |
| Ïðåäìåòíûé óêàçàòåëü |
COD = Cod see “1
Colchicine, synthesis step 146
Collins’ reagent see “Chromium trioxobis(pyridine)-”
Collman’s reagent see “Ferrate(2-) tetracarbonyl-”
Commercial chemicals, inexpensive tables 174—192 263—264 284 290
Complex hydrides see “Aluminum” “Aluminate” “Borane” “Borate”
Configurational selectivity see “Stereoselectivity”
Connection, retro-synthetic 195
Contraction of rings see “Ring contraction”
Convergent growth of dendrimers 354—355
Convergent synthesis 224—225 239—240 280—281 324—329
Cope elimination of tert. amine N-oxides 140
Cope rearrangement of allylcyclohexadienone 319—320
Cope rearrangement, degenerate, of bullvalene 332—333
Cope rearrangement, degenerate, of semibulivalcne 331
Cope rearrangement, oxa- = Claisen rearrangement 87 325
Copper(1+) helicate complexes 345—346
Copper(1+) oxide (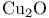 ), oxidative 1,2-bis-decarboxylation 337 ), oxidative 1,2-bis-decarboxylation 337
Copper(1+)/Copper(2+), 1-alkyne coupling with 40 154—155 339
Copper(1+)/Copper(2+), 1-alkynylation of iminium ions with 57
Copper(1-), diorganyl- see “Cuprates(1-)”
Copper(2+), catalyst for oxn. of alkenes to diols with aq. 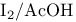 128 128
Copper(2+), oxn. of  -hydroxy carbonyl compounds 133 -hydroxy carbonyl compounds 133
Copper(2+), oxn. of alkenes to  -allyl Pd complexes 27 -allyl Pd complexes 27
Copper(2+), oxn. of hydrazines to diazenes 331
Copper, (1-alkynyl)-, 1-alkynyl d-synthon 339
Copper, (trimethylstannyl)-,  reagent, hydrostannylation of alkynes 321 reagent, hydrostannylation of alkynes 321
Copper, organylbis(tributylphosphine)-, d-synthon 277
Copper/Copper(1+), catalyst for the generation of carbenes 74—75 94
Coproporphyria 256
Copy DNA (cDNA) 242
Corannulene = dibenzo[ghi,mno]fluoranthene 140
Corrins 259—262
Corrins, A/D-bipyrrolidine synthon 66 260
Corrins, methine bridges by S extrusion 59—60 261
Corroles 259
Corroles, reduction of 259
Corticrocin 40
Cortisol, 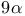 -fluoro- 287 -fluoro- 287
Coupling see “Oxidative coupling” “Nucleotides” “Peptides” “Reductive
Coupling, Pd(0)-catalysed, Heck, alkene + organyl halide 42—43
Coupling, Pd(0)-catalysed, Stille, aryl triflate + organostannane 42 295
CPG = controlled-pore glass particles 221
Cram’s rules 106—107
Crotonaldehyde see “2-Butenal”
Crowding see “Sterical strain due to crowding”
Cryptands 247—248
CSI see “Sulfuryl chloride isocyanate”
Cubane = ![$pentacyclo[4.2.0.0^{2,4}.0^{3,8}.0^{4,7}]octane$](/math_tex/022b3fac6d444780e8fe59c2e96c2ed882.gif) , rearrangements 332 , rearrangements 332
Cubane = ![$pentacyclo[4.2.0.0^{2,4}.0^{3,8}.0^{4,7}]octane$](/math_tex/022b3fac6d444780e8fe59c2e96c2ed882.gif) , synthesis 78 , synthesis 78
Cuneane = ![$pentacyclo[3.3.0.0^{2,4}.0^{3,7}.0^{6,8}]octane$](/math_tex/165ce3bb0c620afc891cb2f46e5b82ee82.gif) 332 332
Cuprates(1-), alkanediyldi-, coupling with 20
Cuprates(1-), diorganyl- 5
Cuprates(1-), diorganyl-, alkylation with, of  -unsaturated carbonyl compounds 20—21 277 -unsaturated carbonyl compounds 20—21 277
Cuprates(1-), diorganyl-, alkylation with, of 2-alkynoic acids 64
Cuprates(1-), diorganyl-, alkylation with, of carboxylic acid halides 46
Cuprates(1-), diorganyl-, alkylation with, of cyclopropyl ketones 276
Cuprates(1-), diorganyl-, alkylation with, of haloalkanes 19—20
Cuprates(1-), diorganyl-, alkylation with, of oxiranes 21
Cuprates(1-), diorganyl-, alkylation with, of oxiranylmethyl mesylates 125
Cuprates(1-), diorganyl-, alkylation with, of thioesters 320
Cuprates(1-), diorganyl-, alkylation with, of vinylic halides 20
Cuprates(1-), diorganyl-, enantioselective reagents 20—21
Cuprates(1-), diorganyl-, relative reactivity of substrates to 19—20
Curtius degradation 139 143 301 308 331.
Cyanide anion,  -synthon 2 6 18 44 50 66 199 301 302 -synthon 2 6 18 44 50 66 199 301 302
Cyanide anion, basicity 6
Cyanide anion, catalyst for the oxidation 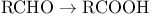 - via acyl cyanides 134—135 - via acyl cyanides 134—135
Cyanoborohydride see “Borate(1-) cyanotrihydro-”
Cyanogen bromide, cleavage of tert. amines 309
Cyanohydrins see “Nitriles
Cyclic compounds see “Carbocycles” “Heterocycles”
Cyclizations see “Carbocycles” “Heterocycles”
Cyclizations, 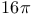 -electrocyclization (thermal antarafacial) 262 -electrocyclization (thermal antarafacial) 262
Cyclizations, 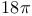 -electrocyclization (thermal antarafacial) 334 -electrocyclization (thermal antarafacial) 334
Cyclizations, 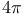 -electrocyclization (thermal antarafacial), vinylcyclopropane-cyclopentene rearr. 77 83 -electrocyclization (thermal antarafacial), vinylcyclopropane-cyclopentene rearr. 77 83
Cyclizations, 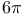 -electrocyclization (photochem. antarafacial or thermal suprafacial) in alkaloid synthesis 295—296 -electrocyclization (photochem. antarafacial or thermal suprafacial) in alkaloid synthesis 295—296
Cyclizations, 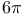 -electrocyclization (photochem. antarafacial or thermal suprafacial), hexatriene-cyclohexadiene rearr. 331—332 -electrocyclization (photochem. antarafacial or thermal suprafacial), hexatriene-cyclohexadiene rearr. 331—332
Cyclizations, 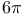 -electrocyclization (photochem. antarafacial or thermal suprafacial), meta-cyclophane cyclization 38 338 -electrocyclization (photochem. antarafacial or thermal suprafacial), meta-cyclophane cyclization 38 338
Cyclizations, acid-catalysed 90—92 93 278—280 309
Cyclizations, butadiene-cyclobutene rearr. (photochem. suprafacial) 78 331
Cyclizations, cyclopropylmethanimine-dihydropyrrole rearr. 298—299
Cyclizations, cyclopropylmethanimine-dihydropyrrole rearr., pentadienylium-cyclopentenylium rearr. 258
Cyclizations, favored ring sizes in porphyrin synthesis 252—253
Cyclizations, favored ring sizes in porphyrin synthesis, stepwise 254—257
Cyclizations, favored ring sizes of butadiene (Ni template) 41
Cyclizations, favored ring sizes of diterminal diynes 40 155 339
Cyclizations, favored ring sizes, acid-catalysed cyclizatioin transforms and 1,5-enynes 90—92 279—281
Cyclizations, favored ring sizes, classic a+d or r+r ring closures 23—24 41 48 52 53—54 55 71—74 81 93 145—146 315—316 318
Cyclizations, favored ring sizes, classic a+d or r+r ring closures, Baldwin rules 315—316
Cyclizations, favored ring sizes, cyclooligomerization of alkynes 80 281 329—330
Cyclizations, favored ring sizes, template cyclizations 248 257 260 262
Cyclizations, [1+2]cycloadditions, aziridination 154 212—213
Cyclizations, [1+2]cycloadditions, cyclopropanation 74—76 94
Cyclizations, [2+2+2]cyclotrimerization of alkynes 80 281 330
Cyclizations, [2+2]cycloadditions,  -lactams 153 -lactams 153
Cyclizations, [2+2]cycloadditions, 1,2-oxaphosphetanes 29—30
Cyclizations, [2+2]cycloadditions, 2,2-dichlorocyclobutanones 83 276
Cyclizations, [2+2]cycloadditions, cyclobutanes 78 297—298 333
Cyclizations, [2+2]cyclodimerization of alkynes 329—330
Cyclizations, [2+3]cycloadditions of 1,3-dipoles to multiple bonds 152—153
Cyclizations, [2+3]cycloadditions of TosMIC to carbonyl groups 49 51
Cyclizations, [2+3]cycloadditions of “trimethylenemethane” to alkenes 84
Cyclizations, [2+4]cycloadditions see “Diels — Alder reaction”
Cycloadditions see “Cyclizations”
Cycloalkane rings see “Carbocycles”
Cyclobutabenzenes see “Bicyclo[4.2.0]octa-1
Cyclobutane derivs. see “Carbocycles 4-membered”
Cyclobutanone, 2-hydroxy-, synthesis 54
Cyclobutanones, synthesis 77 79 335—336
Cyclobutene, synthesis 142
Cyclobutenes, perhalogenated, synthesis 78
Cyclobutenes, perhalogenated, thermal opening 53—54
Cyclocarbons 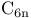 (n=3, 4, 5), MS detection 339 (n=3, 4, 5), MS detection 339
Cyclodecyne, redn. of, E:Z ratio 100—101
Cyclodiphosphathiane see “Dithiadiphosphetane”
Cyclododecanone, pr. 191
Cyclododecanone, reaction with 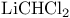 52 52
Cyclododecyne, redn. of, E:Z ratio 100—101
Cyclohexanamines, N-alkylidene-, lithiated 12 56—57
Cyclohexane derivs. see “Carbocycles 6-membered”
Cyclohexanol, 2,3-dimethyl-, pr. 189
Cyclohexanol, 2,3-dimethyl-, synthesis with 206
Cyclohexanol, 5-methyl-2-(1-methylethyl)-, ![$(-)-[1R-(1\alpha,2\beta,5\alpha)]$](/math_tex/e7d12161d0c1e827e3563a12125b9c1582.gif) - (menthol), asymm. induction with 59 - (menthol), asymm. induction with 59
Cyclohexanol, 5-methyl-2-(1-methylethyl)-, ![$(-)-[1R-(1\alpha,2\beta,5\alpha)]$](/math_tex/e7d12161d0c1e827e3563a12125b9c1582.gif) - (menthol), pr. 189 - (menthol), pr. 189
Cyclohexanone, 2-methyl-, pr. 190
Cyclohexanone, 2-methyl-, red. elimn. of tosylhydrazone 141—142
Cyclohexanone, 2-methyl-, regioselective Robinson anellation 73
Cyclohexanone, 2-methyl-, synthesis via enamines 25—26
Cyclohexanone, 2-methyl-, synthesis via SAMP-hydrazone (enantiosel.) 25—26
Cyclohexanone, 2-methyl-, trans-6-alkylation 12
Cyclohexanone, 4-(1,1-dimethylethyl)-, ax-eq selectivity tests with 44—45
Cyclohexanone, 4-(1,1-dimethylethyl)-, pr. 190
Cyclohexanone, 5-methyl-2-(1-methylethylidene)-, (+)-(R)- ((+)-pulegone), pr. 190
Cyclohexanone, 5-methyl-2-(1-methylethylidene)-, (+)-(R)- ((+)-pulegone), pr., Favorskii rearr. of 84 211
Cyclohexanone, 5-methyl-2-(1-methylethylidene)-, (+)-(R)- ((+)-pulegone), pr., reduction of 106
Cyclohexanone, azine = cyclohexylidenehydrazone 35
Cyclohexanone, pr. 190
Cyclohexanone, spiroanellation of 24
Cyclohexanones, oxidative ring opening 82 137 195 206
Cyclohexanones, pr. 190
Cyclohexanones, synth. from alkoxyarenes 87 103—104 278
Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (-)-(S)- (limonene), pr. 190
Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (-)-(S)- (limonene), regioselective reactions, epoxidation 124 156—157
Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (-)-(S)- (limonene), regioselective reactions, hydroboration 130—131
Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (-)-(S)- (limonene), regioselective reactions, hydrogenation 101
Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (-)-(S)- (limonene), regioselective reactions, ozonolysis 87—88 156—157
Cyclohexene, oxidative ring contraction 130
Cyclohexene, pr. 189
Cyclohexenes, ox. opening 81—82 87—89
| Cyclohexenes, ox. ring contraction 81—82 130
Cyclononyne, redn. of, E:Z ratio 100—101
Cyclooctene, (-)-(E)-, synthesis 142
Cyclopentadecanone, 3-methyl- (muscone),  -, synthesis 41 -, synthesis 41
Cyclopentane derivs. see “Carbocycles 5-membered”
Cyclopentanecarboxaldehydes 130
Cyclopentanecarboxylic acids 83—84 211
Cyclopentanecarboxylic acids, 2-oxo-, esters, pr. 189
Cyclopentanecarboxylic acids, 2-oxo-, esters, synthesis by intramolecular condensations 3 55 59 81 82 83
Cyclopentanellations see “Carbocycles 5-membered”
Cyclopentanone in 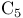 -homologization of carobxylic acids 88 -homologization of carobxylic acids 88
Cyclopentanone, 2-methyl-, enolization 11 63
Cyclopentanone, 2-methyl-, selective 2-alkylation 63
Cyclopentanone, oxime, Beckmann rearr. 136—137
Cyclopentanone, oxime, pr. 189
Cyclopentanone, pr. 189
Cyclopentanones, synthesis 82—84
Cyclopentenes from 1,5-dioxo compds 41
Cyclophanes of porphyrins 253
Cyclopropane derivs. see “Carbocycles 3-membered”
Cyclopropane, 1-bromo-2-methoxy-, cis- and trans-, synthesis 74—75
Cyclopropane, 1-bromo-2-methoxy-, cis-, lithiation and reactions 70 76
Cyclopropane, methylene-, cyclobutanone from 77
Cyclopropane, methylene-, synthesis 74—75
Cyclopropanecarboxylic acids, esters, ring opening 69
Cyclopropanecarboxylic acids, esters, synthesis 74—76
Cyclopropanecarboxylic acids, pr. 189
Cyclopropanecarboxylic acids, synthesis 74—76 136
Cyclopropanes, (1-alkenyl)-, ring expansion 76 77 83
Cyclopropanes, (1-alkenyl)-, synthesis 75
Cyclopropanes, activated, ring opening 14—15 69—70 76—78 276
Cyclopropanols, 1-(haloamino)-, ring expansion to  -lactams 77—78 -lactams 77—78
Cyclopropanone, ketals, ring opening 14—15 70
Cyclopropanone, ketals, synthesis 75
Cyclopropanone, reaction with amines 77—78
Cyclopropanone, reaction with amines, synthesis 75
Cyclopropene, prepn. and cycloaddn. of 92
Cyclopropyl aldimines and ketimines, ring expansion 298—299
Cyclopropyl methyl ketone = 1-cyclopropyl-, ethanone, pr. 189
Cyclopropyl methyl ketone = 1-cyclopropyl-, synthesis 130
Cyclopropylogous (= homovinylogous) principle 15—16 69—70 76—77 288—289
Cycloreversion see “Ring cleavage”
Cyclosteroids 288—289
Cyclotridecanone, 2-hydroxy-, synthesis 54
Cyclotrisilathiane, 2,2,4,4,6,6-hexamethyl-, 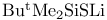 reagent from 169 reagent from 169
Cycloundecyne, redn. of, E:Z ratio 100—101
D-Gluconamide, N-(6,8-tetradecadiynyl)-, aqueous suspension, tubular micelles 353
D-Gluconamide, N-octyl-, aqueous suspension, helical fiber micelles 352
d-Orbitals of Si, P, S etc., carbanion stabilization 6—9
d-Orbitals of Si, P, S, carbanion stabilizn. 6—9
D-Ribonic acid, esters, D-psicosides from 272
d-Synthons see “Donor synthons”
d.e. see “Diastereomeric excess”
Darzens reaction 45
DAST = (N-ethylethanaminato)trifluorosulfur (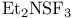 ) 269—270 272 ) 269—270 272
dATP see “2'-Deoxynucleoside 5'-triphosphates”
Davison nickel see “Raney 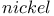 ” ”
DBN see “1
DCG see “Carbodiimide dicyclohexyl-”
dCTP see “2'-Deoxynucleoside 5'-triphosphates”
DDQ see “1 4
Deblocking 154—167
Deblocking of 1,2-diols, acetonides 265 267 277 323
Deblocking of 1,3-diols, acetonides 323
Deblocking of 1,3-diols, benzylidene acetals 268—269 326
Deblocking of 1-alkynes (1-silylated) 155 339
Deblocking of carbon-carbon double bonds,  -iodo ethers 326—327 -iodo ethers 326—327
Deblocking of carbon-carbon double bonds, 1,2-dihalides 119 156—157
Deblocking of carbon-carbon double bonds, 1,2-diols 142
Deblocking of carbon-carbon double bonds, oxiranes 157
Deblocking of carbonyl groups, acetals 130 165—166 291 328—329 339 349
Deblocking of carbonyl groups, alkenes (ozonolysis) 322 323
Deblocking of carbonyl groups, dicyanomethylene derivs. 166
Deblocking of carbonyl groups, dithioacetals 22 51 79 156 165 267 328—329
Deblocking of carbonyl groups, enol ethers 48—49 54 278
Deblocking of carbonyl groups, monothioacetals 76 165—166
Deblocking of carboxy groups 165
Deblocking of carboxy groups, 1,1-dimethylethyl esters 307
Deblocking of carboxy groups, alkyl esters 37 274
Deblocking of carboxy groups, silyl esters 313
Deblocking of enolates (with RLi), enol acetates 57—58
Deblocking of enolates (with RLi), enol silyl ethers 57—58 73
Deblocking of hydroxy groups see “Nucleotides”
Deblocking of mercapto groups 169 229 239—240
Deblocking of methylene groups 109 155—156
Deblocking of phenols, methyl ethers 280
Deblocking of phenols, phenylmethyl ethers 305
Deblocking of phosphates 166—167 216—220 224 342
Deblocking of prim, amino groups (labile amides) 161—164 216—218 224 229 235—240 267 342
Deblocking of sec. amino groups, acetamides 304
Deblocking of sec. amino groups, carbamates 235—236 301
Deblocking of sec. amino groups, sulfonamides 247 304
Deblocking of sec. amino groups, tert. amines (cleavage with BrCN) 309
Deblocking of tert. amino groups, thermal dequaternization 304
Deblocking, acetate esters etc. 157—158 266—269 271 273 282 283
Deblocking, acetate esters etc.,  -iodo ethers 326—327 -iodo ethers 326—327
Deblocking, acetate esters etc., (1,1-dimethylethyl)dimethylsilyl ethers 62 157 159 276—277 281 323 328 329
Deblocking, acetate esters etc., (1,1-dimethylethyl)diphenylsilyl ethers 272
Deblocking, acetate esters etc., (4-methoxyphenyl)methyl (= MPM = PMB) ethers 157 325—329
Deblocking, acetate esters etc., 1,1-dimethylethyl (t-butyl) ethers 280 283
Deblocking, acetate esters etc., 2,2,2-trihaloethyl carbonates 157—158
Deblocking, acetate esters etc., 2-propenyl (allyl) ethers 270
Deblocking, acetate esters etc., 9-phenyl-9H-xanthan-9-yl (pixyl) ethers 342
Deblocking, acetate esters etc., dialkylborinate esters 60—62 67—68
Deblocking, acetate esters etc., enantiotoposelective 167—168 276—277
Deblocking, acetate esters etc., methyl ethers 157 161 275
Deblocking, acetate esters etc., phenylmethyl (benzyl) ethers 157—159 161 229 271 328
Deblocking, acetate esters etc., tetrahydro-2H-pyran-2-yl (THP) ethers 82 157 159—160 273
Deblocking, acetate esters etc., trimethylsilyl ethers 83 159 275 281
Deblocking, acetate esters etc., triphenylmethyl (trityl) and related ethers 216 218—222 224
Decalin see “Naphthalene decahydro-”
Decanoic acid, 10-oxo, methyl ester, olefination of 31
Decarboxylation 139
Decarboxylation of  -oxo carboxylic acids 81—83 139 195 -oxo carboxylic acids 81—83 139 195
Decarboxylation of 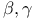 -unsatd. -unsatd. 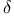 -lactones 80 92 331 -lactones 80 92 331
Decarboxylation of 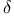 -oxo -oxo 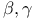 -unsatd. acids 209—210 331 -unsatd. acids 209—210 331
Decarboxylation of diarylacetic acids 303
Decarboxylation, oxidative, of  -amino acids 48 -amino acids 48
Decarboxylation, oxidative, of 1,2-dicarboxylic acids 80 142 333 337
Decarboxylation, oxidative, of carboxylic acids to amines 139 143 301 308 331
Dehydrogenation 122 138—140
Dehydrogenation of porphyrinogens 251—253 349
Dehydrogenation, aromatization, with Pd 337
Dehydrogenation, aromatization, with trityl cation 249 334
Dehydrogenation, exhaustive 139—140 338
Dendrimers = dendritic polymers 354—356
Deoxygenation of N-, P- and S-oxides 115
Deoxyribonucleic acid see “DNA”
Deoxysulfonation, reductive 34
Deprotection see “Deblocking”
Deprotonation see “Bases”
Depurination, undesired side-reaction 222
Desilylation see “Deblocking”
Dess — Martin periodinane (BMP) 134 328—329
Desulfurization see “Sulfones” “Thiiranes” “Thioethers”
Desymmetrization, diastereotoposelectjve 168
Dewar benzenes = bicyclo[2.2.0]hexa-2,5-dienes 330
Dexbrompheniramine 303
Dextropropoxyphene 300
dGTP see “2'-Deoxynucleoside 5'-triphosphates”
DHP see “2H-Pyran 3
Diademane = octahydro-1,2,3-methano-1H-dicycloprop[cd,hi]indene 333
Dialdehydes by ox. cleavage of cycloalkenes 81—82 87—88
Dialdehydes from diones + 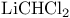 51—52 51—52
Diastereo-zeroplane, definition 360
Diastereofaces, definition 360
Diastereomeric excess = diastereoselectivity, definition, prelim. (footnote d.e. = d.s. = % (l)-isomer-% (u)-isomer) XIII 107
Diastereoselectivity see “Asymmetric induction” “Axial/equatorial-selectivity” “cis/trans-selectivity” “endo/exo-selectivity” “erythro/threo-selectivity” “Inversion” “Retention”
Diastereoselectivity, diastereotopic face selectivity 126—127
Diastereoselectivity, diastereotopic group selectivity 168 360
|
|
 |
| Ðåêëàìà |
 |
|
|
 |
|
Î ïðîåêòå
|
|
Î ïðîåêòå



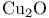 ), oxidative 1,2-bis-decarboxylation
), oxidative 1,2-bis-decarboxylation 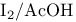
 -hydroxy carbonyl compounds
-hydroxy carbonyl compounds  -allyl Pd complexes
-allyl Pd complexes  reagent, hydrostannylation of alkynes
reagent, hydrostannylation of alkynes 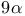 -fluoro-
-fluoro- ![$pentacyclo[4.2.0.0^{2,4}.0^{3,8}.0^{4,7}]octane$](/math_tex/022b3fac6d444780e8fe59c2e96c2ed882.gif) , rearrangements
, rearrangements ![$pentacyclo[3.3.0.0^{2,4}.0^{3,7}.0^{6,8}]octane$](/math_tex/165ce3bb0c620afc891cb2f46e5b82ee82.gif)
 -unsaturated carbonyl compounds
-unsaturated carbonyl compounds  -synthon
-synthon 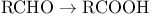 - via acyl cyanides
- via acyl cyanides 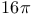 -electrocyclization (thermal antarafacial)
-electrocyclization (thermal antarafacial) 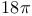 -electrocyclization (thermal antarafacial)
-electrocyclization (thermal antarafacial) 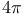 -electrocyclization (thermal antarafacial), vinylcyclopropane-cyclopentene rearr.
-electrocyclization (thermal antarafacial), vinylcyclopropane-cyclopentene rearr. 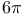 -electrocyclization (photochem. antarafacial or thermal suprafacial) in alkaloid synthesis
-electrocyclization (photochem. antarafacial or thermal suprafacial) in alkaloid synthesis  -lactams
-lactams 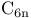 (n=3, 4, 5), MS detection
(n=3, 4, 5), MS detection 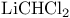
![$(-)-[1R-(1\alpha,2\beta,5\alpha)]$](/math_tex/e7d12161d0c1e827e3563a12125b9c1582.gif) - (menthol), asymm. induction with
- (menthol), asymm. induction with  -, synthesis
-, synthesis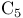 -homologization of carobxylic acids
-homologization of carobxylic acids 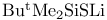 reagent from
reagent from 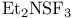 )
)  ”
” -unsatd.
-unsatd.  -lactones
-lactones