|
|
 |
| Àâòîðèçàöèÿ |
|
|
 |
| Ïîèñê ïî óêàçàòåëÿì |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fuhrhop J., Penzlin G. — Organic Synthesis: Concepts, Methods, Starting materials |

Îáñóäèòå êíèãó íà
Íàøëè îïå÷àòêó?
Âûäåëèòå åå ìûøêîé è íàæìèòå Ctrl+Enter
|
Íàçâàíèå: Organic Synthesis: Concepts, Methods, Starting materials
Àâòîðû: Fuhrhop J., Penzlin G.
ßçûê: 
Ðóáðèêà: Õèìèÿ/
Ñòàòóñ ïðåäìåòíîãî óêàçàòåëÿ: Ãîòîâ óêàçàòåëü ñ íîìåðàìè ñòðàíèö
ed2k:
Èçäàíèå: second edition, rewise and enlarged
Ãîä èçäàíèÿ: 1994
Êîëè÷åñòâî ñòðàíèö: 432
Äîáàâëåíà â êàòàëîã: 29.10.2005
Îïåðàöèè: Ïîëîæèòü íà ïîëêó |
Ñêîïèðîâàòü ññûëêó äëÿ ôîðóìà | Ñêîïèðîâàòü ID
|
|
 |
| Ïðåäìåòíûé óêàçàòåëü |
Carbocycles, 7-membered, pr. 191
Carbocycles, 7-membered, synthesis via bicyclo[4.1.0]heptanes 76 334
Carbocycles, 8-membered, pr. 191
Carbocycles, bridged see “Oligocyclic compounds bridged”
Carbocycles, macrocyclic, pr. 191
Carbocycles, macrocyclic, synthesis by 1-alkyne coupling 40 155
Carbocycles, macrocyclic, synthesis by acyloin coupling 53—54
Carbocycles, macrocyclic, synthesis by allyl coupling with Ni 41—42
Carbocycles, macrocyclic, synthesis by butadiene cyclooligomerization 41
Carbocycles, macrocyclic, synthesis by fragmentation of fused systems 89—90
Carbocycles, macrocyclic, synthesis by sulfide contraction 38—39 338
Carbocycles, pr. 189—192
Carbocycles, retro-synthesis 207—211
Carbocycles, synthesis 71—87 90—94 “Ring “Ring
Carbodiimide, dicyclohexyl- (= N,N-meihanetetraylbis[cyclohexanamine]), Moffatt — Pfitzner oxidation with 133
Carbodiimide, dicyclohexyl- (= N,N-meihanetetraylbis[cyclohexanamine]), syntheses with, of aryl ethers 144—145
Carbodiimide, dicyclohexyl- (= N,N-meihanetetraylbis[cyclohexanamine]), syntheses with, of azlactones 48 231
Carbodiimide, dicyclohexyl- (= N,N-meihanetetraylbis[cyclohexanamine]), syntheses with, of esters and amides 144—146 326—327
Carbodiimide, dicyclohexyl- (= N,N-meihanetetraylbis[cyclohexanamine]), syntheses with, of peptides 231—236 240—241
Carbohydrates see “Monosaccharides” “Oligosaccharides”
Carbohydrates, pr. 263—264 (table)
Carbon monoxide, carbonyl synthon 46—48
Carbon monoxide, photolytic elimination or extrusion 330 339
Carbon monoxide, thermal elimination 337
Carbon oxides 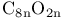 , (n=3, 4, 5) 338—339 , (n=3, 4, 5) 338—339
Carbon-carbon bonds see “Alkanes” “Alkenes” “Alkynes”
Carbonates, alkali hydrogen, deprotonation of ct-acylthio ketones 60
Carbonates, alkali or alkaline-earth,  -eliminations of halides 52 140 -eliminations of halides 52 140
Carbonates, alkali or alkaline-earth, deblocking of O-acyl derivs. 157—158 273
Carbonates, alkali or alkaline-earth, deprotonation of 1,3-diones 10
Carbonates, alkali or alkaline-earth, deprotonation of alkylphosphonium salts 31
Carbonates, alkali or alkaline-earth, deprotonation of phenols 355
Carbonates, alkali or alkaline-earth, epimerization of  -oxy aldehydes 265 -oxy aldehydes 265
Carbonazidic acid, 1,1-dimethyl ester (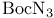 ), protecting reagent 162—163 ), protecting reagent 162—163
Carbonic acid diesters, carboxyln. with 49
Carbonitriles see “Nitriles”
Carbonochloridic acid esters, protecting reagents 157—158 162—163 229
Carbonothioic acid, O,O'- (1,2-alkanediyl) cyclic ester, reductive elimination 142
Carbonothioic acid, O,O'-dialkyl esters, pyroiysis 138 140—141
Carbonyl compounds see “Aldehydes” “Ketones”
Carbonyl compounds,  -unsaturated, -unsaturated,  -synthons 16 19 -synthons 16 19
Carbonyl compounds,  -unsaturated, -unsaturated,  -alkylation of 20—21 22 -alkylation of 20—21 22
Carbonyl compounds,  -unsaturated, cyclopropanation of 75—76 -unsaturated, cyclopropanation of 75—76
Carbonyl compounds,  -unsaturated, reduction of the C=C bond 73 103—104 -unsaturated, reduction of the C=C bond 73 103—104
Carbonyl compounds,  -unsaturated, reduction of the C=O bond 105—107 265 -unsaturated, reduction of the C=O bond 105—107 265
Carbonyl compounds,  -unsaturated, synthesis of, by -unsaturated, synthesis of, by  -dehydrogenation 122 138—140 -dehydrogenation 122 138—140
Carbonyl compounds,  -unsaturated, synthesis of, by 3-eliminations 57 72—73 140 -unsaturated, synthesis of, by 3-eliminations 57 72—73 140
Carbonyl compounds,  -unsaturated, synthesis of, by aldol type reactions 55—59 -unsaturated, synthesis of, by aldol type reactions 55—59
Carbonyl compounds,  -unsaturated, synthesis of, by dimsylation of esters 65 -unsaturated, synthesis of, by dimsylation of esters 65
Carbonyl compounds,  -unsaturated, synthesis of, by oxidation of allylic -unsaturated, synthesis of, by oxidation of allylic  119—120 119—120
Carbonyl compounds, protection see “Protection of carbonyl groups”
Carboperoxoic acids, oxidation with, Baeyer — Villiger type 80 136—137 165 276 319—320
Carboperoxoic acids, oxidation with, of alkenes to oxiranes 18 34 88 117 123—127 156—157
Carboperoxoic acids, oxidation with, of thioethers to sulfones 80
Carboperoxoic acids, oxidation with, of thioethers to sulfoxides 315
Carboperoxoic acids, relative reactivity of 123
Carbosulfenylation of alkenes (Reetz) 21
Carbothioic acids, S-(2-oxoalkyl) esters, S extrusion 59—60
Carbothioic acids, S-(2-pyridyl) or S-(2-imidazolyl) esters, macrocyclic lactones from 146
Carbothioic acids, S-alkyl esters, 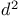 -synthons 62 322—323 -synthons 62 322—323
Carbothioic acids, S-alkyl esters, aldol addition with chiral boron enolates 62
Carbothioic acids, S-alkyl esters, Wittig oleflnation of 32
Carbothioic acids, thionolactones, cyclic ethers from 110—111
Carboxamides,  -unsaturated, Michael addn. to 72 -unsaturated, Michael addn. to 72
Carboxamides, alcoholysis 303
Carboxamides, hydrolysis (mild) 312—313
Carboxamides, N,N-dialkyl-, adducts with 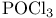 (Vilsmeier reagents) 16 18 255 293 (Vilsmeier reagents) 16 18 255 293
Carboxamides, N-alkylation (Mitsunobu) 160—161
Carboxamides, O-alkylation 111—112
Carboxamides, reduction, to aldehydes 98 105
Carboxamides, reduction, to amines 98 111—112 247 291
Carboxamides, synthesis see “Peptides”
Carboxamides, synthesis by acylation of amines 144—145
Carboxamides, synthesis by acylation of amines with carboxylic acid azides 237—241
Carboxamides, synthesis by acylation of amines with carboxylic acid esters 66 112 234 291 297 299 354
Carboxamides, synthesis by acylation of amines with carboxylic acid halides or mixed anhydrides 247 291 312—313
Carboxamides, synthesis by acylation of amines with carboxylic acids (DCC) 144—145 231—236 240—241 326—327
Carboxamides, synthesis by acylation of amines, catalysts for 144—145 231 234
Carboxamides, synthesis by hydrolysis of nitriles 66 302—304
Carboximidic acids, esters, reactions, as 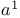 -synthons 260 -synthons 260
Carboximidic acids, esters, reactions, as 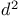 -synthons 18—19 22—23 63—64 197—198 -synthons 18—19 22—23 63—64 197—198
Carboximidic acids, esters, reduction of, to aldehydes 13 22—23 63—64 298
Carboximidic acids, esters, reduction of, to sec. amines 111—112
Carboximidic acids, esters, synthesis from carboxamides 111—112 260
Carboximidic acids, esters, synthesis from nitriles 22—23 298
Carboximidoyl chlorides, N-alkyl-, nitrile ylides from 153
Carboximidoyl chlorides, N-hydroxy-, nitrile N-oxides from 153
Carboxylic acids,  -alkyl- -alkyl- -hydroxy-, stereosel. synth. 27 62 -hydroxy-, stereosel. synth. 27 62
Carboxylic acids,  -alkyl-, synthesis 13 22—23 199—201 299 -alkyl-, synthesis 13 22—23 199—201 299
Carboxylic acids,  -amino- see “ -amino- see “ -Amino acids” -Amino acids”
Carboxylic acids,  -hydroxy-, synthesis by -hydroxy-, synthesis by  -oxn. of acids 120—122 -oxn. of acids 120—122
Carboxylic acids,  -hydroxylation 120—121 -hydroxylation 120—121
Carboxylic acids,  -unsaturated -unsaturated  -acylamino-, enantioselective hydrogenation 102—103 -acylamino-, enantioselective hydrogenation 102—103
Carboxylic acids,  -unsaturated, esters, Michael addition to 65—66 351 -unsaturated, esters, Michael addition to 65—66 351
Carboxylic acids,  -unsaturated, esters, red. coupling with carbonyl compounds 69 -unsaturated, esters, red. coupling with carbonyl compounds 69
Carboxylic acids,  -unsaturated, oxidative decarboxylation ( -unsaturated, oxidative decarboxylation (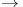 ketones) 143 ketones) 143
Carboxylic acids,  -unsaturated, synthesis by aldol type condensations 56 -unsaturated, synthesis by aldol type condensations 56
Carboxylic acids,  -unsaturated, synthesis from 2-alkynoic acids by -unsaturated, synthesis from 2-alkynoic acids by  -alkylation 64 -alkylation 64
Carboxylic acids,  -unsaturated, synthesis from 2-alkynoic acids by cis-hydrogenation 64 -unsaturated, synthesis from 2-alkynoic acids by cis-hydrogenation 64
Carboxylic acids,  -hydroxy-, erythro- -hydroxy-, erythro- -alkylation 27 325—326 -alkylation 27 325—326
Carboxylic acids,  -hydroxy-, synthesis, aldol type addition 59 61—62 -hydroxy-, synthesis, aldol type addition 59 61—62
Carboxylic acids,  -hydroxy-, synthesis, enantioselective (Noyorl) hydrogenation of -hydroxy-, synthesis, enantioselective (Noyorl) hydrogenation of  -oxo acids 102—103 325—326 -oxo acids 102—103 325—326
Carboxylic acids,  -hydroxy-, synthesis, Reformatsky reaction 44—45 301 -hydroxy-, synthesis, Reformatsky reaction 44—45 301
Carboxylic acids,  -oxo-, esters, -oxo-, esters,  -acidity 10 -acidity 10
Carboxylic acids,  -oxo-, esters, cyclopentanes from 83 -oxo-, esters, cyclopentanes from 83
Carboxylic acids,  -oxo-, esters, decarboxylation 81 82—83 139 195 205 209—210 -oxo-, esters, decarboxylation 81 82—83 139 195 205 209—210
Carboxylic acids,  -oxo-, esters, enantiosel, hydrogenation 102—103 325—326 -oxo-, esters, enantiosel, hydrogenation 102—103 325—326
Carboxylic acids,  -oxo-, esters, reduction to satd. esters 114—115 -oxo-, esters, reduction to satd. esters 114—115
Carboxylic acids,  -oxo-, esters, synthesis by ester condensation 55 58—59 -oxo-, esters, synthesis by ester condensation 55 58—59
Carboxylic acids,  -oxo-, esters, synthesis, -oxo-, esters, synthesis,  -enol ethers from 2-alkynoic acids 63 -enol ethers from 2-alkynoic acids 63
Carboxylic acids, 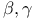 -unsaturated, synthesis from enones by Favorskii rearrangement 84 -unsaturated, synthesis from enones by Favorskii rearrangement 84
Carboxylic acids,  -hydroxy- (and -hydroxy- (and  -lactones) 63 64—65 69 -lactones) 63 64—65 69
Carboxylic acids,  -oxo-, esters, synthesis from enol ether + diazoacetic ester 69—70 -oxo-, esters, synthesis from enol ether + diazoacetic ester 69—70
Carboxylic acids,  -oxo-, esters, synthesis from nitroalkane + 2-enoic acid 65—66 -oxo-, esters, synthesis from nitroalkane + 2-enoic acid 65—66
Carboxylic acids,  -oxo-, esters, synthesis from Trost’s reagent + ketone 79 -oxo-, esters, synthesis from Trost’s reagent + ketone 79
Carboxylic acids, 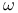 -hydroxy, lactones from cyclic ketones 80 136—137 206 319—320 -hydroxy, lactones from cyclic ketones 80 136—137 206 319—320
Carboxylic acids, 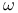 -oxo-, synthesis, hydroformylation of co-enoic acids 47—48 -oxo-, synthesis, hydroformylation of co-enoic acids 47—48
Carboxylic acids, 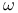 -oxo-, synthesis, reduction of cyclic anhydrides 46—47 -oxo-, synthesis, reduction of cyclic anhydrides 46—47
Carboxylic acids, 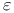 -oxo-, synthesis by oxidative ring opening from cyclohexanol ethers 87—88 206 -oxo-, synthesis by oxidative ring opening from cyclohexanol ethers 87—88 206
Carboxylic acids, 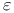 -oxo-, synthesis by oxidative ring opening from cyclohexanones 137 -oxo-, synthesis by oxidative ring opening from cyclohexanones 137
Carboxylic acids, 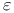 -oxo-, synthesis by oxidative ring opening from cyclopentanone + RCOCl 88 -oxo-, synthesis by oxidative ring opening from cyclopentanone + RCOCl 88
Carboxylic acids, 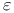 -oxo-, synthesis by oxidative ring opening from tm-cyclohexanols 136 -oxo-, synthesis by oxidative ring opening from tm-cyclohexanols 136
Carboxylic acids, azides, peptide synthesis with 237—241
Carboxylic acids, azides, preparation 143 237—241 301
Carboxylic acids, azides, racemization of, lack of 231
Carboxylic acids, azides, rearr. to isocyanates 143 301
Carboxylic acids, conversion to functional derivs. 143—146 (see also “Carboxamides” etc.)
Carboxylic acids, conversion to ketones with RLi 45—46 283
Carboxylic acids, degradation to amines 139 143 301 308 331
Carboxylic acids, derivatives 143—146
Carboxylic acids, dianions, prepn. 63
Carboxylic acids, esters see “Lactones”
Carboxylic acids, esters,  -acidity 10 -acidity 10
Carboxylic acids, esters,  -alkylation 22 27 319—320 -alkylation 22 27 319—320
Carboxylic acids, esters,  -hydroxylation 121—122 -hydroxylation 121—122
Carboxylic acids, esters, 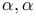 -dialkylation (asym.) 22 -dialkylation (asym.) 22
Carboxylic acids, esters, hydrazides and azides from 239—240 301
Carboxylic acids, esters, hydrolysis see “Deblocking”
Carboxylic acids, esters, methylenation with Tebbe’s reagent 110 272
Carboxylic acids, esters, O-silylation of enolates 325
Carboxylic acids, esters, oxidation to  -unsaturated esters 139 -unsaturated esters 139
Carboxylic acids, esters, pyroiysis 140—141
Carboxylic acids, esters, reaction with DMSO anion 50—51 65
Carboxylic acids, esters, reduction to aldehydes 105—106 159 165 203 272—273 276 283 321 322
Carboxylic acids, esters, reduction to ethers 110—111
Carboxylic acids, esters, reduction to prim. alcohols 98 325 326 349
Carboxylic acids, esters, reductive coupling (acyloin coupling) 53—54
Carboxylic acids, esters, synthesis, carboxylation with 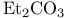 49 49
Carboxylic acids, esters, synthesis, esterification of alcohols 144—146 347
Carboxylic acids, esters, synthesis, esterification of alcohols, inversion-esterification 160—161 286
| Carboxylic acids, esters, synthesis, hydration of 1-alkoxy-1-alkynes 64—65 325 327—328
Carboxylic acids, esters, synthesis, oxidation of aldehydes 134—135
Carboxylic acids, esters, synthesis, oxidation of ethers 118 134—135
Carboxylic acids, esters, synthesis, oxidative rearrangement, Baeyer — Villiger 136—137 282 283
Carboxylic acids, esters, synthesis, oxidative rearrangement, skeletal rearr. of ketones 136
Carboxylic acids, halides, conversion to  -functionalized ketones 50—51 -functionalized ketones 50—51
Carboxylic acids, halides, conversion to azides 143
Carboxylic acids, halides, conversion to esters 144
Carboxylic acids, halides, conversion to ketones 46—47
Carboxylic acids, halides, preparation 143
Carboxylic acids, halides, reduction to aldehydes 46 96—98
Carboxylic acids, halides, umpolung with 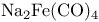 17 46—47 17 46—47
Carboxylic acids, protection see “Protection of carboxy groups”
Carboxylic acids, reduction to prim. alcohols 98
Carboxylic acids, synthesis by oxidation of alcohols 134 319—320
Carboxylic acids, synthesis by oxidation of aldehydes 134
Carboxylic acids, synthesis by oxidative cleavage of  -hydroxy ketones 62 -hydroxy ketones 62
Carboxylic acids, synthesis by oxidative cleavage of alkynes 117 132
Carboxylic acids, synthesis by retro-aldol type cleavage 88 207
Carboxylic acids, synthesis from anions with 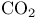 49 64 200 49 64 200
Carboxylic acids, synthesis via propanediol acids 139 199—200
Carcerand 356
Cardenolides, glycosylation 267
Cardenolides, synthesis 286—287
Carotenoids, synthesis 30—31 40—41
Carvone 1,2-oxide, ![$[(1S)-1\alpha,2\alpha,4\beta]$](/math_tex/bc95c4902701308f4a55ea4da2cf11c682.gif) -, chiral educt 282 -, chiral educt 282
Carvone, (-)-(R)- and (+)-(S)-, pr. 191
Cascade-branched polymers 354—356
Catalysts see “Hydrogenation” “Lewis “Sulfonic
Catalysts, aldol addition, stereoselective 61
Catalysts, amidation/esterification 144—146 231 234
Catalytic hydrogenation see “Hydrogenation”
Catechol derivatives see “1
Catecholborane = 1,3,2-benzodioxaborole 131
Cation-exchange resins see “Sulfonic acids”
Cavitands 355
Cbz (benzyloxycarbonyl) 162 163
cDNA = copy DNA 242
Ce (2-cyanoethyl), phosphate protecting group 166—167 216 218 220—224 342
Cephalexin 312—313
Cephalosporanic acid, 7-amino- 312—313
Cephalosporin C 312—313
Cephalosporin syntheses, partial 312—313
Cephalosporin syntheses, total 313—315
Ceramide, glycosylation of 271
Cerate(2-), hexanitrato-, diammonium (CAN, ceric ammonium nitrate,  ), oxn. of (4-methoxyphenyl)methyl ethers 157 ), oxn. of (4-methoxyphenyl)methyl ethers 157
Cerate(2-), hexanitrato-, diammonium (CAN, ceric ammonium nitrate,  ), oxn. of iron(0) complexes 78 ), oxn. of iron(0) complexes 78
Cerebrosides 271
Cerium(3+) chloride + 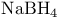 , reduction with 105—106 107 , reduction with 105—106 107
Chelation stereocontrol 60—62 66—67
Chichibabin reaction, amination of pyridine derivs. 306
Chiral auxiliaries see “Enantioselective syntheses”
Chiral bilayer effect 352
Chiral hydrogenation catalysts 102—103
Chiral induction see “Asymmetric induction”
Chirality, self-reproduction of 299
Chloranil see “Cyclohexadienedione tetrachloro-”
Chlordiazepoxide 307
Chlorins 257—259
Chloro-acetoxylation of diols 160
Chloroalkanes see “Haloalkanes”
Chlorochromate, pyridinium see “Chromate(1-)”
Chlorophyll a 257
Chlorophyll a, synthesis 258—259
Chlorophyll b, formula 257
Chlorothiazide 310
Chlorpheniramine, (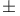 )- 303 )- 303
Cholecalciferol (vitamin 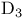 ) 31—32 ) 31—32
Cholecalciferol (vitamin 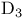 ), ),  -dihydroxy- 281—283 -dihydroxy- 281—283
Cholest-5-en-3-ol,  - (cholesterol), 17-fluorination 118—119 156—157 - (cholesterol), 17-fluorination 118—119 156—157
Cholest-5-en-3-ol,  - (cholesterol), degradation of the - (cholesterol), degradation of the 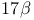 side-chain 118—119 side-chain 118—119
Cholest-5-en-3-ol,  - (cholesterol), pr. 284 - (cholesterol), pr. 284
Cholesta-5,7-dien-3-ol,  - (provitamin - (provitamin 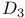 ), electrocyclic ring opening 289 ), electrocyclic ring opening 289
Cholesta-5,7-dien-3-ol,  - (provitamin - (provitamin 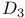 ), pr. 284 ), pr. 284
Cholestan-3-ol, 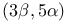 -, remote oxidation 285—286 -, remote oxidation 285—286
Chromate(1-), chlorotrioxo-, pyridinium (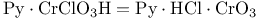 , PCC), oxn. of sec. alcohols to ketones 283 , PCC), oxn. of sec. alcohols to ketones 283
Chromate(2-), potassium (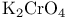 ), oxidation of allylic ), oxidation of allylic 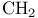 120 120
Chromic acid bis(1,1-dimethylethyl) ester, oxidation with, of alcohols to aldehydes 133—135
Chromic acid bis(1,1-dimethylethyl) ester, oxidation with, of allylic 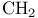 116 120 116 120
Chromium(2+) acetate, reduction of oxiranes 274—275
Chromium(6+) oxide with acetic acid, oxidative cleavage of tert. alcohols 136
Chromium(6+) oxide with sulfuric acid (Jones’ reagent), oxn. with, of alkanes 118—119
Chromium(6+) oxide with sulfuric acid (Jones’ reagent), oxn. with, of enol ethers 285
Chromium(6+) oxide with sulfuric acid (Jones’ reagent), oxn. with, of sec. alcohols 109 111 117 133—135 276—277 337
Chromium, trioxobis(pyridine)- (Collins’ reagent,  , ,  ), allylic oxidation of alkenes 116 120 ), allylic oxidation of alkenes 116 120
Chromium, trioxobis(pyridine)- (Collins’ reagent,  , ,  ), oxn. of prim, alcohols 125 ), oxn. of prim, alcohols 125
Chromium, trioxobis(pyridine)- (Collins’ reagent,  , ,  ), oxn. of sec. alcohols (selective) 135 ), oxn. of sec. alcohols (selective) 135
Chugaev pyroiysis of thioesters 138 140—141
Cinchona alkaloids, chiral 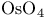 -oxidation catalysts from 129 -oxidation catalysts from 129
Cinchona alkaloids, pr. 290
cis/trans-Isomerization of alkenes 30—31 100—101 142 282 296 332
cis/trans-Selective reactions see “Inversion” “Retention”
cis/trans-Selective reactions,  -selectivity, -selectivity,  -alkylation of cyclic ketones 12 82 -alkylation of cyclic ketones 12 82
cis/trans-Selective reactions,  -selectivity, -selectivity,  -alkylation of lactones 321 322 327—328 -alkylation of lactones 321 322 327—328
cis/trans-Selective reactions,  -selectivity, -selectivity,  -hydroxyln. of carbonyl compds 120—122 -hydroxyln. of carbonyl compds 120—122
cis/trans-Selective reactions,  -selectivity, acid-catalysed cyclization of 1,5-dienes 91—92 279—280 -selectivity, acid-catalysed cyclization of 1,5-dienes 91—92 279—280
cis/trans-Selective reactions,  -selectivity, allylsilane addn. to 2-enones (Sakurai) 90 -selectivity, allylsilane addn. to 2-enones (Sakurai) 90
cis/trans-Selective reactions,  -selectivity, chloro-acetoxylation of diols 160 -selectivity, chloro-acetoxylation of diols 160
cis/trans-Selective reactions,  -selectivity, halohydrination 117 275—276 287 -selectivity, halohydrination 117 275—276 287
cis/trans-Selective reactions,  -selectivity, halolactonization 275 319—320 -selectivity, halolactonization 275 319—320
cis/trans-Selective reactions,  -selectivity, Heck coupling 43 -selectivity, Heck coupling 43
cis/trans-Selective reactions,  -selectivity, hydration of cyclic enones 274—275 -selectivity, hydration of cyclic enones 274—275
cis/trans-Selective reactions,  -selectivity, hydroboration 131 -selectivity, hydroboration 131
cis/trans-Selective reactions,  -selectivity, Ireland — Claisen rearr. 87 324—325 -selectivity, Ireland — Claisen rearr. 87 324—325
cis/trans-Selective reactions,  -selectivity, iron-cyclohexadienyl complexes 44 -selectivity, iron-cyclohexadienyl complexes 44
cis/trans-Selective reactions,  -selectivity, lactonization (diastereosel.) 168 327—328 -selectivity, lactonization (diastereosel.) 168 327—328
cis/trans-Selective reactions,  -selectivity, pinacol coupling of dioxo compds 53—54 -selectivity, pinacol coupling of dioxo compds 53—54
cis/trans-Selective reactions,  -selectivity, redn. of cyclic ketones 106—107 274—275 -selectivity, redn. of cyclic ketones 106—107 274—275
cis/trans-Selective reactions,  -selectivity, [1+2]cycloadditions 74—76 -selectivity, [1+2]cycloadditions 74—76
cis/trans-Selective reactions,  -selectivity, [2+2]cycloadditions 29—30 78 -selectivity, [2+2]cycloadditions 29—30 78
cis/trans-Selective reactions,  -selectivity, [2+3]cycloadditions 84 -selectivity, [2+3]cycloadditions 84
cis/trans-Selective reactions,  -selectivity, [2+4]cycloadditions 84—86 280—281 297 -selectivity, [2+4]cycloadditions 84—86 280—281 297
cis/trans-Selective reactions,  -selectivity, “ene” reactions 282—283 297—298 -selectivity, “ene” reactions 282—283 297—298
cis/trans-Selective reactions, anti/syn-Selectivity, 1,2-diols from alkenes 81—82 117 123—129
cis/trans-Selective reactions, anti/syn-Selectivity, reduction of alkenes by Birch reduction 73 103—104 278
cis/trans-Selective reactions, anti/syn-Selectivity, reduction of alkenes with boranes 89—90 107—108 130—131
cis/trans-Selective reactions, anti/syn-Selectivity, reduction of alkenes with diazene 102
cis/trans-Selective reactions, anti/syn-Selectivity, reduction of alkenes with hydrogen 96—97 101—102 209
cis/trans-Selective reactions, E/Z-Selectivity, enolate formation 12—13 22—23 26 27 57—58 60—62
cis/trans-Selective reactions, E/Z-Selectivity, fragmentation of fused ring systems 89—90
cis/trans-Selective reactions, E/Z-Selectivity, Heck coupling 43
cis/trans-Selective reactions, E/Z-Selectivity, hydrozirconation/bromination 132 325
cis/trans-Selective reactions, E/Z-Selectivity, Julia — Lythgoe olefination 34
cis/trans-Selective reactions, E/Z-Selectivity, reduction of alkynes by Birch reduction 97 100—101
cis/trans-Selective reactions, E/Z-Selectivity, reduction of alkynes with boranes 37—38
cis/trans-Selective reactions, E/Z-Selectivity, reduction of alkynes with DIBAL 100
cis/trans-Selective reactions, E/Z-Selectivity, reduction of alkynes with hydrogen 64 96—97 100—102
cis/trans-Selective reactions, E/Z-Selectivity, reductive coupling of alkynes via 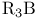 37—38 37—38
cis/trans-Selective reactions, E/Z-Selectivity, reductive coupling of vinylic halides via 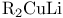 20 277 20 277
cis/trans-Selective reactions, E/Z-Selectivity, ring opening of cyclopropanes 70—71 76—77
cis/trans-Selective reactions, E/Z-Selectivity, sulfur extrusion 39 261
cis/trans-Selective reactions, E/Z-Selectivity, Wittig/Horner olefinations 29—31 273 276 281 283 329
Citronellal, pr. 174
Citronellal, synthesis starting from 206
Claisen condensation see “Ester condensation”
Claisen rearrangement, Ireland- 87 325
Clathrochelates 247—248
Cleavage see “Decarboxylation” “Retro-...”
Cleavage of rings see “Ring cleavage” “Ring
Cleavage, oxidative see “Oxidative cleavage”
Cleavage, reductive see “Hydrogenolysis” “Reductive
Cleavage, site-specific, of DNA, by restriction endonucleases 243
Cleavage, site-specific, of DNA, by synthetic “scissors” 343—344
Clemmensen reduction 109
Clidinium bromide,  - 309 - 309
Cloning of genes 241—246
Cobalt(2+) chloride, catalyst for the reduction of prim. amides with 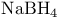 111—112 111—112
Cobalt, 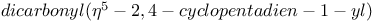 -, alkyne cyclotrimerization catalyst 80 281 -, alkyne cyclotrimerization catalyst 80 281
Cobyrinic acid 259
Cobyrinic acid, synthesis 261—262
|
|
 |
| Ðåêëàìà |
 |
|
|
 |
|
Î ïðîåêòå
|
|
Î ïðîåêòå



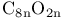 , (n=3, 4, 5)
, (n=3, 4, 5)  -eliminations of halides
-eliminations of halides  -oxy aldehydes
-oxy aldehydes 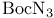 ), protecting reagent
), protecting reagent  -unsaturated,
-unsaturated, 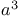 -synthons
-synthons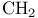
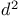 -synthons
-synthons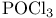 (Vilsmeier reagents)
(Vilsmeier reagents) 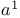 -synthons
-synthons 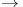 ketones)
ketones) 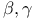 -unsaturated, synthesis from enones by Favorskii rearrangement
-unsaturated, synthesis from enones by Favorskii rearrangement  -hydroxy- (and
-hydroxy- (and 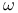 -hydroxy, lactones from cyclic ketones
-hydroxy, lactones from cyclic ketones 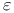 -oxo-, synthesis by oxidative ring opening from cyclohexanol ethers
-oxo-, synthesis by oxidative ring opening from cyclohexanol ethers 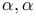 -dialkylation (asym.)
-dialkylation (asym.) 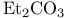
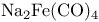
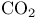
![$[(1S)-1\alpha,2\alpha,4\beta]$](/math_tex/bc95c4902701308f4a55ea4da2cf11c682.gif) -, chiral educt
-, chiral educt  ), oxn. of (4-methoxyphenyl)methyl ethers
), oxn. of (4-methoxyphenyl)methyl ethers 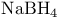 , reduction with
, reduction with  )-
)-  )
)  -dihydroxy-
-dihydroxy-  - (cholesterol), 17-fluorination
- (cholesterol), 17-fluorination 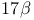 side-chain
side-chain 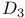 ), electrocyclic ring opening
), electrocyclic ring opening 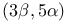 -, remote oxidation
-, remote oxidation 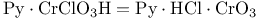 , PCC), oxn. of sec. alcohols to ketones
, PCC), oxn. of sec. alcohols to ketones 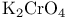 ), oxidation of allylic
), oxidation of allylic  ,
,  ), allylic oxidation of alkenes
), allylic oxidation of alkenes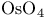 -oxidation catalysts from
-oxidation catalysts from  -selectivity,
-selectivity, 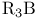
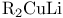
 -
- 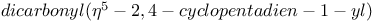 -, alkyne cyclotrimerization catalyst
-, alkyne cyclotrimerization catalyst