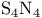Авторизация
Поиск по указателям
Greenwood N.N., Earnshaw A. — Chemistry of the Elements
Обсудите книгу на научном форуме Нашли опечатку?
Название: Chemistry of the ElementsАвторы: Greenwood N.N., Earnshaw A. Аннотация: When this innovative textbook first appeared in 1984 it rapidly became a great success throughout the world and has already been translated into several European and Asian languages. Now the authors have completely revised and updated the text, including more than 2000 new literature references to work published since the first edition. No page has been left unaltered but the novel features which proved so attractive have been retained. The book presents a balanced, coherent and comprehensive account of the chemistry of the elements for both undergraduate and postgraduate students. This crucial central area of chemistry is full of ingenious experiments, intriguing compounds and exciting new discoveries. The authors specifically avoid the term `inorganic chemistry' since this evokes an outmoded view of chemistry which is no longer appropriate in the final decade of the 20th century.
Язык: Рубрика: Химия /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Издание: second editionГод издания: 1997Количество страниц: 1340Добавлена в каталог: 18.02.2007Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
Cluster compounds, boron carbide 149 Cluster compounds, boron hydrides 151—180 Cluster compounds, carbido metal carhonyls 1107—1108 Cluster compounds, carboranes 181—189 Cluster compounds, cobalt, rhodium and indium carbonyls 928ff 1140—1143 Cluster compounds, cobalt-sulfur complexes 1119 Cluster compounds, gold phosphines 1197 Cluster compounds, iron, ruthenium and osmium carhonyls 928 1104—1108 Cluster compounds, lanthanide halides 1242 Cluster compounds, lithium alkyls 103—105 Cluster compounds, lithium imides 100 Cluster compounds, mercury-containing 1120 Cluster compounds, metal borides 148 149—151 Cluster compounds, metallocarboranes 189—195 Cluster compounds, metallohoranes 171—173 178 Cluster compounds, molyhdenum and tungsten dihalides 1022 Cluster compounds, nickel, palladium and platinum carbonyls 1168—1171 Cluster compounds, niobium and tantalum halides 991 Cluster compounds, of phosphorus, arsenic, antimony and bismuth 563 588 Cluster compounds, of tellurium 761 764 Cluster compounds, rhenium alkyls 1068 1069 Cluster compounds, rhenium carbidocarbonyls 1065 Cluster compounds, scandium halides 950 Cluster compounds, stabilization by encapsulated heteroatoms 950 966 992 1065 1107 1141. 1242 Cluster compounds, technethun and rhenium chalcogenides 1049 Cluster compounds, tungsten and molyhdenum halides 1021—1022 Cluster compounds, zirconium halides 965 Cobalt, abundance JII3 Cobalt, allyl complexes 933 Cobalt, arsenide 555 556 Cobalt, atomic and physical properties 1115—1116 Cobalt, biochemistry of 1138 1139 1322 Cobalt, carbidocarbonyls 1141—1142 Cobalt, carbonyls 928 929 1140—1143 Cobalt, complexes, +1 oxidation state 1133 Cobalt, complexes, +2 oxidation state 1129—1133 Cobalt, complexes, +3 oxidation state 1122—1129 Cobalt, complexes, +4 oxidation state 1121 Cobalt, complexes, +5 oxidation state 1121 Cobalt, complexes, lower oxidation states 1137 Cobalt, complexes, with 701 Cobalt, complexes, with S 666—669 Cobalt, coordination numbers and stereochemistries 1117 Cobalt, cyclobutadiene complex 936 Cobalt, cyclooctatetraene complexes 942 Cobalt, cyclopenladienyls 1143 Cobalt, dithiolene complexes, redox series 676 Cobalt, halides 1119—1121 Cobalt, importance of 914 1122 1123 1302 Cobalt, nitrate (anhydrous) 469 Cobalt, nitrato complexes 469 470 543 Cobalt, optical resolution of $mathrm{[Co{(\mu-OH)_2Co(NH_3)_4}_3} 670 915 Cobalt, organomelallic compounds 1139—1143 Cobalt, oxidation states 1117 Cobalt, oxides 1117—1119 Cobalt, oxoanions 1120 1121 Cobalt, production and uses 1114 1115 Cobalt, reactivity of element 1116 Cobalt, relationship with other transition elements 1116—1117 Cobalt, standard reduction potentials 1122 Cobalt, sulfides 1118 Cobaltile (cobalt glance) 1114 Cobaltocene 939 1143 Coesite 342 343 Coinage metals (Cu, Ag, Au) 1173 see Coke 274 see Coke, historical importance is steel making 1070 1072 Cold fusion (nuclear) 1280 1283—1284 Columbite 977 Columhium 976 see Combustion 600—602 612 Complexometric titration of 577 Contact process see “Sulfuric acid manufacture” Cooper pairs 1183 Cooperativity 11000 1199 Coordinate bond 198 921 see Coordination number 912 Coordination number, above nine 917 Coordination number, eight 916 Coordination number, five 914 Coordination number, four 913 Coordination number, nine 917 Coordination number, seven 916 Coordination number, six 916 Coordination number, three 913 Coordination number, two 945 Copper oxide 642 Copper, abundance 1174 Copper, acetylide 1180 Copper, alkenes and alkynes 1199 Copper, alkyls and aryls 1200 Copper, biochemistry of 1197 Copper, carhoxylates 1192—1193 Copper, chalcogenides 1181—1182 Copper, complexes, +1 oxidation state 1194—1197 Copper, complexes, +2 oxidation stale 1189-1194 Copper, complexes, +3 oxidation stale 1187 Copper, Cu-S-O system 677 Copper, halides 1183—1187 Copper, history 1173 Copper, nitrate, structure of 471 1190 Copper, nitrato complexes 469 470 471 544 Copper, organometallic compounds 925 1199 1200 Copper, oxides 1181 Copper, production and uses 1174 1175 see Corundum see “Aluminium oxides” Cosmic black-body radiation 2 Cossee “mechanism 261 Cotton effect 1125 Creutz — Taube anion 1097 Cristobalite 343 344 Critical mass 1256 1257 1261 Crocidolite 351 Crocoite 1003 Crown ethers 96 97 Crown ethers, complexes with alkali metals 95 97 Crown ethers, complexes with alkaline earth metals 124 Crown ethers, “hole sizes” of 96 Crown ethers, “triple-decker” complex Cryolite 219 Crypt, complexes with alkali metals 97 393 394 Crypt, complexes with alkaline earth metals 125 Crypt, molecular structure of 98 Crystal field splittings 922—923 Crystal field stabilization energy 1131 Crystal field theory 922 Crystal field, octahedral 922 Crystal field, strong 923 Crystal field, weak 922 Cuhic, eight coordination 916 1275 1480 Cupellation 1173 Cuprite 1174 Curium 1252 1262 see Cyanamide 319 Cyanamide, industrial production 324 Cyanates 320 324 Cyanates, as ligands 325 Cyanide ion as ligand 322 926 Cyanide process 1175 1196 Cyanogen 319—321 Cyanogen, halides 320 323 340 Cyanuric acid 305 Cyanuric compounds 320 323 Cyclo polyphosphoric acids 529 see Cyclo- 656 Cyclo- 656—657 Cyclo- 654 Cyclo- 654—656 Cyclo- 660 Cyclo- 654 Cyclo- 654 Cyclo- 660 Cyclo-polyphosphates 529—531 Cyclo-silicates 347 349 Cyclobutadiene as 935—938 Cycloheptatrienyl as 941 Cyclometaphosphoric acids $\mathrm{(HРo_3)_n} 512 see “Cydo-poly-phospates” Cycloocta-l,5-diene (cod) as ligand 932 Cyclooctatetraene as 942 Cyclooctatetraene as 943 Cyclopentadienyl, as 940 see individual Cyclopentadienyl, as 937—940 Cyclophosphazanes 533 534 Cycto- 657 Cycto- 656—657 Cycto- 657 Cycto- 656—658 Cycto- 656 658 778 Cycto- 656 659 Cydo-polyarsanes 584-586 Cydometaphosphimic acids 541 542 Cytochrames 1095 1101 1198 1279 Cytosine 61. 62 Cаtеnа-polyarsanes 584—587 d orbitals 922—923 1285—1289 d orbitals, splitting by crystal fields 922—923 d-block contraction 27 222 251 561. 1234 Dalton's atomic theory 509 Dalton's law of multiple proportions 509 Dawson structure 1015 Decaborane, 159 163 Decaborane, 176 Decaborane, 175 Decaborane, 175—177 Decaborane, 187 Decaborane, 175 Degussa process for HCN 321 Density of the elements, periodic trends in 24 Denticity 906 Deoxyribonucleic acids 476 Detergents, polyphosphates in 474 477 Detergents, sodium tripolyphosphate in 528 Deuterium, atomic properties 34 Deuterium, diwovery 32 Deuterium, ortho- and para- 36 Deuterium, physical properties 35 Deuterium, preparation 39 Dewar — Chatt — Duncanson theory 931 Diagonal relationship 27 Diagonal relationship, B and Si 202 347 Diagonal relationship, Be and Al 107 Diagonal relationship, Li and Mg 76 102 1113 Diagonal relationship, N and S 722 Diamond, chemical properties 278 Diamond, occurrence and distribution 271 272 Diamond, physical properties 278 Diamond, production and uses 272 Diarsane, 583 see Diaspore 243 Diatomaceous earth 342 Diatomic molecules (homonuclear), bond dissociation, energies of 584 Diazotization of aromatic amines 463 Dibenzenechromium 940 1039 see Diborane, 154 159 Diborane, 163—170 Diborane, 165 Diborane, 166 183 Diborane, 164 Diborane, 164 Dibromonium cation 569 Dicacodyl, 583—585 Dichlonne hexoxide, 844 845 849 850 Dichlorine monoxide, 844—847 Dichromate ion, 1009 Dicyandiamide 320 324 Dielectric constant, influence of H bonding 55 Dihydrogen 34 Dihydrogen, coordination chemistry of 44—47 Dimethyl suit oxide as ionizing solvent 694 Dimethylaminophosphorus dihalides 533 Dinicrogen monoxide see “Nitrous oxide” Dinitrogen pentoxide 444 458 Dinitrogen tetroxide 444 454—458 Dinitrogen tetroxide, chemical reactions 456—458 Dinitrogen tetroxide, nonaqueous solvent properties 456—458 Dinitrogen tetroxide, physical properties 456 457 Dinitrogen tetroxide, preparation 456 Dinitrogen tetroxide, structure 455 see Dinitrogen trioxide, 444 Dinitrogen, as ligand 408 413—416 Dinitrogen, complexes, synthesis of 413 414 Dinitrogen, coordination modes 415 Dinitrogen, discovery of donor properties 414 1097 Dinitrogen, isoelectronic with CO, 416 Diopside 349 Dioxygen see “Oxygen” “Oxygen “Singlet” Dioxygen, bonding in metal complexes 619—620 Dioxygen, chemical properties 612 613 Dioxygen, coordination chemistry of 615 Dioxygen, difluoride 639 Dioxygen, molecular-orbital diagram 606 Dioxygen, paramagnetic behaviour 601 Dioxygen, reaction with haemoglohin 614 1099—1101 Dioxygen, reactions of coordinated 619—620 Dioxygen, singlet state 607 614 716 Dioxygen, singlet-triplet transitions 606 Dioxygen, superoxo and peroxo complexes 615 616 Dioxygen, Vaska's discovery of reversible coordination 615 1135 Diphosphazenes 535 Diphosphonic acid see “Diphosphorous acid” Diphosphoric acid, 510 516 518 Diphosphorous acid, 512 Diphosphorus tetrahalides 497 Disilenes 362 Disulfates, 705 712 Disulfates, 743 Disulfates, 700 Disulfites, 705 720 Disulfuric acid, 705 711 Disulfurous acid, 853 705 720 Dithiocarbamates 317 Dithiocarbamates, as ligands 665 673 674 796 Dithiolenes as ligands 665 674—676 Dithionates, $\mathrm{S_2O_6^{2-}} 705 715 Dithionic acid, 705 715 Dithionites, 705 720 Dithionous acid, 705 720 DNA see “Deoxyribonucleic acids” Dobereiner's triads 21 Dodecahedral complexes 916 Dolomite 109 272 Donor-acceptor complexes see “Class-a and class-b metal ions” “Coordinate “Ligands” individual Donor-acceptor complexes Of 235—237 Donor-acceptor complexes of 552 564 Donor-acceptor complexes of 321 322 Donor-acceptor complexes of 232 Donor-acceptor complexes of 673 673 714 Donor-acceptor complexes of 414—416 1097 Donor-acceptor complexes Of 615—620 Donor-acceptor complexes of 493—495 Donor-acceptor complexes of 495 497 Donor-acceptor complexes Of 561 569 570 702 Donor-acceptor complexes of 324—327 345 Donor-acceptor complexes of 686 Donor-acceptor complexes of 703 704 Donor-acceptor complexes of 723 Donor-acceptor complexes of 665—672 Donor-acceptor complexes of CO see “Carbonyls” Donor-acceptor complexes of cyclo-polyarsanes 584—586 Donor-acceptor complexes of cyclo-polyphosphazenes 540 Donor-acceptor complexes of dithiocarbamates and xanthates 673 674 1080
Реклама
 |
|
О проекте
|
|
О проекте




 in early coordination chemistry
in early coordination chemistry 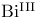 with EDTA
with EDTA  , nonstoichiometry in
, nonstoichiometry in 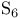 (
(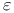 -sulfur)
-sulfur) 
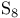 (
( ), crystal and molecular structure
), crystal and molecular structure 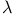 point
point 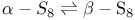
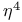 ligand
ligand 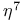 ligand
ligand 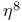 ligand
ligand 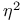 ,
, 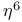 etc., ligand
etc., ligand  ligand
ligand 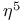 ligand
ligand 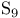
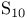
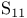
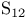
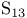
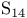

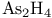
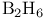
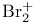 , compound with
, compound with 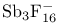
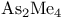
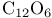
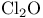
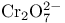
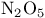
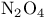
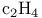
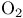
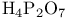
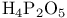
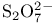

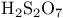
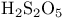
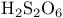
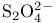
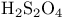
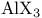
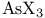


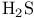
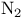
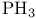 and tertiary phosphines
and tertiary phosphines