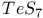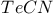јвторизаци€
ѕоиск по указател€м
Greenwood N.N., Earnshaw A. Ч Chemistry of the Elements
ќбсудите книгу на научном форуме Ќашли опечатку?
Ќазвание: Chemistry of the Elementsјвторы: Greenwood N.N., Earnshaw A. јннотаци€: When this innovative textbook first appeared in 1984 it rapidly became a great success throughout the world and has already been translated into several European and Asian languages. Now the authors have completely revised and updated the text, including more than 2000 new literature references to work published since the first edition. No page has been left unaltered but the novel features which proved so attractive have been retained. The book presents a balanced, coherent and comprehensive account of the chemistry of the elements for both undergraduate and postgraduate students. This crucial central area of chemistry is full of ingenious experiments, intriguing compounds and exciting new discoveries. The authors specifically avoid the term `inorganic chemistry' since this evokes an outmoded view of chemistry which is no longer appropriate in the final decade of the 20th century.
язык: –убрика: ’ими€ /—татус предметного указател€: √отов указатель с номерами страниц ed2k: ed2k stats »здание: second edition√од издани€: 1997 оличество страниц: 1340ƒобавлена в каталог: 18.02.2007ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
ѕредметный указатель
Stannocene 402 Starch/iodine reaction 790 864 Stars, spectral classification of 5 Stars, temperatures of 5 Steel 1072Ч1075 Stellar evolution 5 Stereochemical non-rigidity (fluxional behaviour), 230 Stereochemical non-rigidity (fluxional behaviour), 914 1104 Stereochemical non-rigidity (fluxional behaviour), 498 Stereochemical non-rigidity (fluxional behaviour), 684 Stereochemical non-rigidity (fluxional behaviour), 5-coordinate compounds 914 Stereochemical non-rigidity (fluxional behaviour), 8-coordinate compounds 995 Stereochemical non-rigidity (fluxional behaviour), allyl complexes 934 Stereochemical non-rigidity (fluxional behaviour), Berry pseudorotation mechanism 474 499 Stereochemical non-rigidity (fluxional behaviour), iron cyclopentadienyl complexes 1111 Stereochemical non-rigidity (fluxional behaviour), titanium cyclopentadienyl 974 Stibinidene complexes 597 Stibnite see УAntimony sulfideФ УSb_{2}S_{3}Ф Stishovite 342 Strength of oxoacids, Pauling's rules 50 Strontium see also УAlkaline earth metalsФ Strontium, history 108 Strontium, organometallic compounds 136 Strontium, polysulfides 681 Styx numbers see УBoranesФ Sulfamic acid, 408 741 742 Sulfamide 742 743 Sulfanes 682Ч683 (see also УHydrogen sulfideФ) Sulfanes, nonexistence of 685 Sulfanes, physical properties 683 Sulfanes, preparation 682 Sulfanes, synthesis of polythionic acids from 716 Sulfate ion as ligand ( 712 Sulfates 711 712 Sulfide minerals, geochemical classification 648 Sulfide minerals, names and formulae 649 Sulfides, 631 681 Sulfides, anionic polysulfides 678 681 682 Sulfides, applications and uses 677Ч679 Sulfides, electrical properties 681 Sulfides, hydrolysis 678 679 682 Sulfides, industrial production 678 Sulfides, magnetic properties 681 Sulfides, preparation (laboratory) 677 Sulfides, roasting in air 676Ч678 Sulfides, solubility in water 678 679 Sulfides, structural chemistry 679Ч681 Sulfinates 703 Sulfites, 705 719 Sulfites, 652 Sulfites, 719 Sulfoxylates, MS(O)OR 703 Sulfur see also УChalcogenidesФ Sulfur as ligands 701Ч703 Sulfur chloride pentafluoride 687 Sulfur chloride pentafluoride 640 Sulfur chloride pentafluoride 688 689 Sulfur dioxide 698Ч701 (see also УWackenroder's solutionФ УSulfuric Sulfur dioxide as ligand 701Ч703 Sulfur dioxide in MЧSЧO phase diagrams 677 Sulfur dioxide, atmospheric pollution by 646 698Ч700 699 Sulfur dioxide, chemical reactions 700 Sulfur dioxide, clathrate hydrate 700 Sulfur dioxide, industrial production 698 708 Sulfur dioxide, insertion into MЧC bonds 702 703 Sulfur dioxide, molecular and physical properties 700 780 Sulfur dioxide, solvent for chemical reactions 662 701 Sulfur dioxide, toxicity 700 Sulfur dioxide, uses 700 Sulfur imides, 735Ч735 Sulfur in biological complexes 667 Sulfur trioxide see also УSulfuric acid productionФ Sulfur trioxide, chemical reactions 703 704 Sulfur trioxide, molecular and physical properties 703 704 Sulfur trioxide, monomeric 703 704 Sulfur trioxide, polymeric 703 704 Sulfur trioxide, polymorphism 703 704 Sulfur trioxide, preparation by catalytic oxidation of 700 708 Sulfur trioxide, reaction with 640 Sulfur trioxide, reaction with 695 Sulfur trioxide, trimeric 703 704 Sulfur, see УPolyatomic cationsФ Sulfur, 656 661 Sulfur, 656 661 Sulfur, 661 Sulfur, see УPolyatomic cationsФ Sulfur, see УCyclo- Sulfur, see УPolyatomic cationsФ Sulfur, see УCyclo- Sulfur, 660 Sulfur, abundance 647 Sulfur, allotropes 646 652Ч661 Sulfur, atomic properties 661 Sulfur, atomic S 664 Sulfur, atomic weight, variability of 18 661 662 Sulfur, bromides 691 Sulfur, catenation 652 656ff 681Ч683 689 690 716Ч718 Sulfur, chemical reactivity 662Ч664 Sulfur, chiral helices 660 Sulfur, chlorides 689Ч692 716 Sulfur, chlorides, 693 Sulfur, chlorides, 691 693 Sulfur, chlorides, industrial applications of 690 Sulfur, chlorides, preparation 690 691 Sulfur, chlorides, properties of 690 691 Sulfur, chlorofluorides 640 686Ч689 Sulfur, conformations (c, dt, lt) 656 659 665 Sulfur, conversion to see УSulfuric acidФ Sulfur, coordination geometries 663 Sulfur, Crystex 659 Sulfur, dihedral angles in 654 655 Sulfur, fibrous ( 659Ч660 Sulfur, fluorides 683Ч689 Sulfur, fluorides, chemical reactions 685Ч689 Sulfur, fluorides, fluxionality of 685 Sulfur, fluorides, isomeric 684 Sulfur, fluorides, physical properties 685 687 Sulfur, fluorides, stoichiometries 684 Sulfur, fluorides, structures 684Ч685 Sulfur, fluorides, synthesis 685Ч688 Sulfur, gaseous species 661 Sulfur, halides 683Ч693 Sulfur, hexafluoride 685 687 Sulfur, hexafluoride, applications as a dielectric gas 687 Sulfur, hexafluoride, reaction with 695 Sulfur, history 645 646 Sulfur, iodides 691Ч693 Sulfur, iodides, 693 Sulfur, iodides, 692 Sulfur, iodides, 692 693 Sulfur, iodides, 692 Sulfur, iodides, bond energy relations in 691 Sulfur, ligand properties of 665Ч669 668 671 Sulfur, ligand properties of chelating 665 670 672 Sulfur, ligand properties of S atom 665Ч666 Sulfur, liquid 654 660 Sulfur, monoxide 698 Sulfur, nitrides 722Ч729 Sulfur, organic thio ligands 673 Sulfur, origin in caprock of salt domes 647 Sulfur, oxidation state diagram of species 706 Sulfur, oxidation states 664 Sulfur, oxides 695Ч704 (see also УSulfur dioxideФ УSulfur Sulfur, oxides, higher, 704 Sulfur, oxides, lower dioxides 695Ч698 Sulfur, oxides, lower oxides 695Ч698 Sulfur, oxoacids 706Ч721 Sulfur, oxofluorides 688 (see also УThionyl fluoridesФ УSulfuryl Sulfur, peroxofluorides 689 Sulfur, plastic ( 659 Sulfur, polyatomic cations 664 665 Sulfur, polymeric ( 659 Sulfur, production 649Ч652 Sulfur, production, Frasch process 649Ч650 Sulfur, production, from pyrite 651 Sulfur, production, from sour gas and crude oil 651 Sulfur, production, statistics 762 768 Sulfur, radioactive isotopes 661 Sulfur, reserves 651 Sulfur, rhombohedral see Sulfur, rubbery S 659 Sulfur, S-S bonds 652 654 662 667 681Ч683 716Ч718 Sulfur, schematic classification 707 Sulfur, singlet state 661 Sulfur, SN compounds 686 721Ч746 У У УSЧNЧX УSulfur УSЧNЧO УSulfur-nitrogen УSulfur-nitrogen Sulfur, standard reduction potentials of S species 706 Sulfur, table of 705 Sulfur, terrestrial distribution 647 Sulfur, thermodynamic interrelations 706 Sulfur, triplet state 661 Sulfur, uses 651 653 Sulfur, uses of radioactive 661 714 Sulfur, volt-equivalent diagram of S species 706 Sulfur-nitrogen anions, 733Ч734 Sulfur-nitrogen cations, 730Ч733 Sulfur-nitrogen-halogen compounds 736Ч740 Sulfur-nitrogen-halogen compounds, 738 Sulfur-nitrogen-halogen compounds, 738 Sulfur-nitrogen-halogen compounds, 739 Sulfur-nitrogen-halogen compounds, cyclo- 736Ч738 Sulfur-nitrogen-halogen compounds, thiazyl halides NSX 736Ч738 Sulfur-nitrogen-oxygen compounds 740 Sulfur-nitrogen-oxygen compounds, amides of 741 (see also УSulfamic acidФ УSulfamideФ) Sulfur-nitrogen-oxygen compounds, hydrazine derivatives of 743 Sulfur-nitrogen-oxygen compounds, hydroxylamine derivatives of 743Ч746 Sulfur-nitrogen-oxygen compounds, imido and nitrido derivatives of 743 Sulfur-nitrogen-oxygen compounds, sulfur-nitrogen oxides 740 741 Sulfuric acid 706 712 Sulfuric acid, 710 711 Sulfuric acid, amides of 741Ч743 Sulfuric acid, autoprotolysis in anhydrous 710 Sulfuric acid, contact process 646 700 708Ч710 981 Sulfuric acid, history 646 708 Sulfuric acid, hydrates 710 Sulfuric acid, hydrazine derivatives of 744 Sulfuric acid, hydroxylamine derivatives of 744Ч746 Sulfuric acid, imido derivatives of 743 744 Sulfuric acid, ionic dissociation equilibria in anhydrous 711 Sulfuric acid, lead chamber process 646 708 Sulfuric acid, nitrido derivatives of 743 744 Sulfuric acid, production from sulfide ores 708 Sulfuric acid, production from sulfur 652Ч652 708 Sulfuric acid, production statistics 407 708 710 Sulfuric acid, solvent system 711 Sulfuric acid, uses 710 Sulfurous acid, 652 700 705 717 718 Sulfuryl chloride 694 695 Sulfuryl fluoride 688 694 Sulfuryl fluoride, mixed fluoride halides 694 Superconductivity in Chevrel phases 1018 1031 Superconductivity of metal sulfides 680 Superconductivity, high temperature 945 1182Ч1183 1232 Superconductivity, use of Nb/Zr in magnets 978 Superheavy elements 30 1253 Supernucleophiles 1139 Superoxo complexes of 616 1127 Superphosphate fertilizer 474 525 Swarts reaction 560 Symmetry elements 1290Ч1292 Symmetry operations 1290Ч1292 Synergic bonding see also УBack ( Synergic bonding in alkene complexes 926 927 Synergic bonding in CO and 931 Synergic bonding in cobalt cyanides 1122 Synthesis gas 1106 Talc 109 Tanabe Ч Sugano diagrams for 997 Tanabe Ч Sugano diagrams for 1029 Tanabe Ч Sugano diagrams for 1096 1128 Tanabe Ч Sugano diagrams for 1156 Tanabe Ч Sugano diagrams for 1156 Tantalates 987 Tantalite 977 Tantalum see also УGroup 5 elementsФ Tantalum, abundance 977 Tantalum, alkyls and aryls 999 Tantalum, carbene complex 926 Tantalum, carbonylate anions 999Ч1000 Tantalum, chalcogenides 987 Tantalum, complexes, +4 oxidation state 944Ч996 Tantalum, complexes, +5 oxidation state 994 Tantalum, compounds with oxoanions 993 Tantalum, cyclopentadienyls 1000Ч1001 Tantalum, discovery 976 Tantalum, halides and oxohalides 988Ч999 Tantalum, oxides 929 982 983 999Ч1000 Tantalum, production and uses 977 Technetates 1050 Technetium see also УGroup 7 elementsФ Technetium, abundance 1041 Technetium, carbonyls 928 1062Ч1063 Technetium, chalcogenides 1049 Technetium, complexes, +2 oxidation state 1058 Technetium, complexes, +3 oxidation state 1057Ч1058 Technetium, complexes, +4 oxidation state 1056 Technetium, complexes, +5 oxidation state 1055 Technetium, complexes, +6 oxidation state 1055 Technetium, complexes, +7 oxidation state 1054 Technetium, complexes, lower oxidation states 1061 Technetium, cyclopentadienyls 1067Ч1068 Technetium, discovery 1040 Technetium, halides and oxohalides 1051Ч1054 Technetium, nuclear medicine, role in 1042 Technetium, organometallic compounds 1062Ч1067 Technetium, oxides 1045 Technetium, production and uses 1041 Tectites 394 Tecto-silicates 414Ч416 347 Teflon (PTFE) 304 791 Tellurates 782 Telluric acid, 782 Tellurides 765 766 Tellurites 781 Tellurium, abundance 748 Tellurium, allotropy 751 Tellurium, atomic and physical properties 753 754 Tellurium, chemical reactivity 754Ч759 Tellurium, coordination geometries 756Ч757 Tellurium, dioxide 779 780 Tellurium, discovery 747 Tellurium, halide complexes 776 Tellurium, halides 767Ч776 Tellurium, hydride, 759 766 Tellurium, nitrate 786 Tellurium, nitride, 783 Tellurium, organo compounds 786Ч788 Tellurium, oxides 779Ч780 Tellurium, oxoacids 781Ч783 Tellurium, oxohalides 777 Tellurium, polyatomic anions 762Ч765 Tellurium, polyatomic cations, 759 761 Tellurium, polyatomic cations, 761 Tellurium, production and uses 748 749 Tellurium, redox properties 755Ч756 Tellurium, sulfide, 783 Tellurium, toxicity 759 Tellurium, trioxide 780 Tellurocyanate ion, 779 Telluropolythionates 783 Tellurous acid 781
–еклама
 |
|
ќ проекте
|
|
ќ проекте



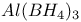 ,
, 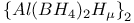
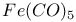
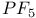
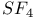
![$H[H_{2}NSO_{3}]$](/math_tex/e64f64d6c43711a812aa0fe004b22c7f82.gif)
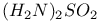
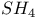 and
and 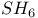
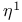 ,
, 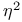 ,
,  )
)  structures
structures 
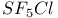 , photoJytic reduction to
, photoJytic reduction to 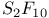
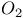
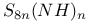
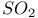
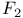
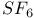
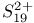
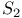
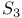
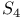
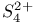
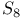
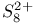
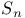
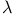 point
point 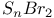
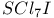
![$[SCl_{3}]^{+}$](/math_tex/8bcf408701794657b0ca225ef4c1a3d382.gif)
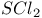 and
and 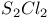
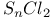
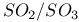 for
for 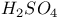
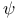 ,
, 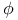 )
) 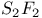
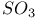
![$[S_{14}I_{3}]^{3+}$](/math_tex/59792cf0b4c2666ced62a9311b76eefd82.gif)
![$[S_{2}I_{4}]^{2+}$](/math_tex/431f483edd0f3f0aca6d62cae9513c8a82.gif)
![$[S_{7}I]^{+}$](/math_tex/5b9ead0c95c50693a51b05ef1c23d68682.gif)
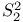
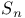
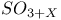 ,
, 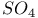
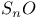
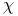 )
) 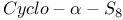
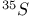
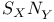
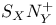
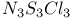
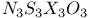
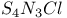 and
and 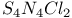
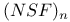
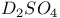
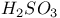
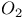
 ) bondingФ
) bondingФ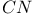 complexes
complexes 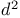 ions
ions 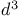 ions
ions 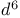 ions
ions 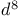 ions
ions 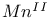
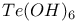
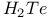
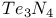
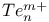
![$[Te_{n}Se_{4Чn}]^{2+}$](/math_tex/3ba284cabf8e1a2aebfc069150c1fff582.gif)