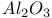Авторизация
Поиск по указателям
Greenwood N.N., Earnshaw A. — Chemistry of the Elements
Обсудите книгу на научном форуме Нашли опечатку?
Название: Chemistry of the ElementsАвторы: Greenwood N.N., Earnshaw A. Аннотация: When this innovative textbook first appeared in 1984 it rapidly became a great success throughout the world and has already been translated into several European and Asian languages. Now the authors have completely revised and updated the text, including more than 2000 new literature references to work published since the first edition. No page has been left unaltered but the novel features which proved so attractive have been retained. The book presents a balanced, coherent and comprehensive account of the chemistry of the elements for both undergraduate and postgraduate students. This crucial central area of chemistry is full of ingenious experiments, intriguing compounds and exciting new discoveries. The authors specifically avoid the term `inorganic chemistry' since this evokes an outmoded view of chemistry which is no longer appropriate in the final decade of the 20th century.
Язык: Рубрика: Химия /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Издание: second editionГод издания: 1997Количество страниц: 1340Добавлена в каталог: 18.02.2007Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
Rhodium, cyclopentadienyls 1143 Rhodium, discovery 1113 Rhodium, halides 1119—1121 Rhodium, optical resolution of 670 Rhodium, organometallic compounds 1139—1143 Rhodium, oxidation states 1117 Rhodium, oxides 1117 1118 Rhodium, production and uses 1114 Rhodium, reactivity of element 1116 Rhodium, relationship with other transition elements 1119 1295 Rhodium, sulfides 1118 Rhodocene 1143 Ribonucleic acids 476 Ring-laddering 99 Ring-stacking 99 RNA 476 Rochelle salt, discovery of ferroelectricity in 57 963 Roussin's salts 447 1094 Rubidium see also “Alkali metals” Rubidium, abundance 70 Rubidium, compounds with oxygen 70—71 Rubidium, discovery 69 Rubridoxins 1098 1101 1102 Ruby 242 1003 Ruby, laser 1029 Russell — Saunders coupling 1242 Rusting of iron 1076 Ruthenates 1082 Ruthenium red 1091 Ruthenium, abundance 1071 Ruthenium, atomic and physical properties 1074—1076 Ruthenium, bipyridyl complexes and solar energy conversion 1096 Ruthenium, blue 1097 Ruthenium, carbidocarbonyls 1107—1108 Ruthenium, carbonyl 928 1104—1105 Ruthenium, carbonyl halides 1108 Ruthenium, carbonyl hydrides and carbonylate anions 1105—1108 Ruthenium, chalcogenides 1081 Ruthenium, complexes, +2 oxidation state 1091—1097 Ruthenium, complexes, +3 oxidation state 1088 1091 Ruthenium, complexes, +4 oxidation state 1086—1088 Ruthenium, complexes, +5 oxidation state 1086 Ruthenium, complexes, +6 oxidation state 1085 Ruthenium, complexes, +7 oxidation state 1085 Ruthenium, complexes, +8 oxidation state 1085 Ruthenium, complexes, with 702 Ruthenium, complexes, with S 668—670 Ruthenium, coordination numbers and stereochemistries 1078 Ruthenium, discovery 1070 Ruthenium, halides 1082—1084 Ruthenium, mixed valence compounds of 1097 Ruthenium, nitrosyl complexes 1097 Ruthenium, organometallic compounds 1104—1287 Ruthenium, oxidation states 1077 1078 Ruthenium, oxides 1079 1080 1255 Ruthenium, oxoanions 1081 Ruthenium, production and uses 1073 Ruthenium, reactivity of element 1075 Ruthenium, relationship to other transition elements 1075—1079 Ruthenium, standard reduction potentials 1077 Ruthenocene 937 1111 Rutherfordium 1281—1282 Rutile 955 961 1119 Rutile, structure type 962 S-N heterocycles incorporating a third element 736 737 s-process in stars 12 Salt (NaCl), history 790 792 Salt (NaCl), location of deposits 793 795 Salt (NaCl), uses in chemical industry 71 72 Saltpetre 407 (see also “Potassium nitrate”) Samarium 1228 (see also “Lanthanide elements”) Samarium, +2 oxidation state 1239 1240 1241 1248 Samarium, magnetic properties 1243 Scandium see also “Group 3 elements” Scandium as eka-boron 944 Scandium, abundance 945 Scandium, complexes 950—953 Scandium, discovery 944 1228 Scandium, halides 949 950 Scandium, organometallic compounds 953 Scandium, oxide 949 Scandium, production 945 Scandium, salts with oxoanions 949 Scheelite 1003 1004 1169 Schoenites 1190 Scope 273 Scotch hearth process for roasting PbS 677 Seaborgium 1281—1283 Secondary valency 912 Selenates 781 Selenic acid, 782 Selenides 765 766 Selenites and diselenites 781 Selenium, abundance 748 Selenium, allotropy 761—753 Selenium, atomic and physical properties 753 754 Selenium, chemical reactivity 754—759 Selenium, coordination geometries 756—757 Selenium, dioxide 779 780 Selenium, discovery 747 Selenium, halide complexes 776 Selenium, halides 767 768 772 Selenium, hydride, 759 766—767 Selenium, nitride, 783 Selenium, organocompounds 759 786 787 Selenium, oxides 779—780 Selenium, oxoacids 781—783 Selenium, oxohalides 777 910 Selenium, polyatomic anions 762—765 Selenium, polyatomic cations, 759—761 Selenium, production and uses 748 749 Selenium, pseudohalides 778 779 911 Selenium, redox properties 755 Selenium, sulfate 786 Selenium, sulfides 783 Selenium, toxicity 759 Selenium, trioxide 780 Selenocyanate ion 329 324—325 Selenocyanate ion, ambidentate properties 757 778 Selenopolythionates 783 Selenosulfates 783 Selenous acid, 781 Semiconductors, As, Sb and Bi chalcogenides 581 679 Semiconductors, III—V 221 255 258 549 Semiconductors, II—VI 255 Semiconductors, nonstoichiometric oxides 644 SHIP technique 1283 SI units inside back cover, conversion to non SI units 1293 Siderite 1071 Siderophile elements 648 Silaethenes 362 Silaneimines 361 Silanes, chemical reaction 338—339 Silanes, homocyclic polysilanes 363 Silanes, physical properties 337 Silanes, silyl haiides 339 340 Silanes, silyl potassium 339 340 Silanes, synthesis 337 Silanethiones 360 Silenes 362 Silica see also “Quartz” “Tridymite” “Cristobalite” “Coesite” “Stishovite” Silica in transistor technology 383 Silica, fumed 345 Silica, gel 345 Silica, historical importance 328 Silica, hydrated 346 Silica, phase diagram 344 Silica, polymorphism 342—346 Silica, uses 345 346 Silica, vitreous 344 Silicate minerals 347—359 Silicate minerals, comparison with silicones 364 Silicates 328—330 347—359 Silicates with chain structures (metasilicates) 349 350 Silicates with discrete units 347—348 Silicates with framework structures 354—359 Silicates with layer structures 349—357 Silicates, disilicates 348 Silicates, metasilicates 348 350 Silicates, orthosilicates 347 348 Silicates, soluble (Na, K) 344 346 Silicides 336—337 Silicides, preparation 336 Silicides, structural units in 337 Silicomanganese 1041 Silicon, abundance and distribution 329 Silicon, atomic properties 330 371 Silicon, carbide 334 Silicon, chemical properties of 328 331 372 Silicon, coordination numbers 335 Silicon, dioxide see “Silica” Silicon, double bonds to 362 Silicon, halides 340—342 Silicon, history 328 Silicon, hydrides see “Silanes” Silicon, isolation 329 330 Silicon, nitride 360 Silicon, organic compounds 361—366 Silicon, physical properties 330 371 372 Silicon, purification 330 Silicon, sulfide 359 Silicones 364—366 Silicones, comparison with mineral silicates 364 Silicones, elastomers 365 Silicones, oils 365 Silicones, organotin, curing agents for 400 Silicones, resins 365 Silicones, synthesis 364 365 Silicones, uses 365 Siloxanes 364 366 Silver halides in photographic emulsions 1186 Silver, abundance 1174 Silver, acetylide 1180 Silver, alkenes and alkynes 1199 Silver, chalcogenides 1181—1182 Silver, complexes, +1 oxidation state 1195 1196 Silver, complexes, +2 oxidation state 1189 Silver, complexes, +3 oxidation state 1187 1188 Silver, halides 1183—1185 Silver, history 1173 Silver, nitrate, thermolysis of 469 Silver, organometallic compounds 1199—1200 Silver, oxides 1181 Silver, production and uses 1174 Silylamides 360 361 Singlet oxygen 607 Singlet oxygen, generation of 614 Singlet oxygen, reactions of 615 Skutterudite see “Cobalt arsenide” Smalt 1113 Smaltite 1114 1145 Soapstone see “Talc” Sodalite see “Ultramarines” Sodanitre see “Chile saltpetre” Sodide anion, 99 Sodium see also “Alkali metals” Sodium chloride structure 80 242 983 Sodium hypochlorite, industrial uses 860 Sodium hypochlorite, Raschig synthesis of hydrazine using 427 428 Sodium, 249 250 Sodium, 678 Sodium, abundance 69 Sodium, arsenide 554 Sodium, azide 409 453 440 Sodium, bismuthate 554 Sodium, carbonate, hydrates of 88 89 104 Sodium, carbonate, production and uses of 89 Sodium, chlorate 862 Sodium, compounds with oxygen 84—86 Sodium, diphosphates 526 527 Sodium, discovery 68 Sodium, distribution 69 70 Sodium, dithionite 721 722 Sodium, hydroxide, production and uses of 72 89 Sodium, hypophosphite 513 Sodium, nitrate, thermolysis of 468 469 Sodium, nitroprusside 447 Sodium, nitroxylate 459 Sodium, orthonirate 471 Sodium, phosphates 512 521 523 524—525 Sodium, polysulfides 677—679 681 688 Sodium, polythionates, preparation and structure 717—718 Sodium, production of metal 71 Sodium, silicates, soluble 343 346 Sodium, solutions in liquid ammonia 77—79 393 Sodium, sulfate, production and uses of 89 Sodium, sulfide batteries 678 679 Sodium, thiosulfate, in photography 714 1186 1187 Sodium, tripolyphosphate 527 528 Solar energy conversion 1096 Solvay process 71 Solvay process, byproduct Ca from 112 Solvo-acids and bases in anhydrous 711 Solvo-acids and bases in liquid 560 Solvo-acids and bases in liquid 831 Solvo-acids and bases in liquid 425 Solvo-acids and bases in liquid 457 Solvo-acids and bases in water 628 Soro-silicates 347—349 Spallation 14 Spectral sensitization of photographic emulsions 1186 Spectroscopic terms 1242 Sphalerite (Zinc blende) 649 1202 Sphalerite (Zinc blende), structure of 679 1209 Spiegeleisen 1041 Spin crossover see “Spin equilibria” Spin equilibria in 1034 Spin equilibria in 1096 Spin equilibria in 1095 Spin equilibria in 1066—1067 Spin equilibria in niobium halides 992 Spin quantum number 22 Spin-forbidden bands in compounds of 1089 Spin-forbidden bands in compounds of 1060 Spin-forbidden bands in compounds of 1158 Spin-orbit coupling in 1087 Spin-orbit coupling in actinide ions 1271 Spin-orbit coupling in lanthanide ions 1242 Spin-orbit coupling in octahedral 1158 Spin-orbit coupling in tetrahedral 1132 Spinel 109 Spinel structure in 1118 Spinel structure in 1079 Spinel structure in 1048 Spinel structure in ferrites and garnets 1081 Spinel structure in ternary sulfides 681 Spinel structure, defect structure of 243 Spinel structure, normal and inverse 247—249 1080 Spinel structure, valence disordered types 249 Spodumene 69 349 Square antiprismatic complexes 916 Square planar complexes 913 Square pyramidal complexes 914 Stability constants of coordination compound, factors affecting 908—911 Stability constants of coordination compound, overall 908 Stability constants of coordination compound, stepwise 908 Staging see “Graphite intercalation compound” Standard reduction potentials 434 Standard reduction potentials, IUPAC sign convention 436 Stannates 354
Реклама
 |
|
О проекте
|
|
О проекте



![$cis-[Rh{\{}{\eta}^{2}-(NH)_{2}SO_{2}{\}}_{2}(OH)_{2})_{2}]^{—}$](/math_tex/5d478abeb7a9c5ce179577029169c90182.gif)
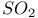
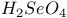
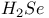
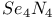
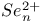 ,
, ![$[Te_{n}Se_{4—n}]^{2+}$](/math_tex/3ba284cabf8e1a2aebfc069150c1fff582.gif)
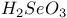
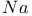
 -alumina, structure and properties
-alumina, structure and properties 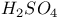
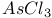 ,
, 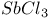
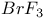
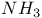
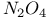
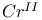 and
and 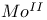 compounds
compounds 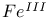 compounds
compounds 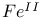 compounds
compounds 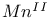 compounds
compounds 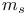
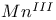
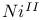
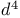 ions
ions 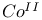
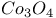
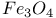 (inverse)
(inverse) 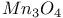
 -
-