Авторизация
Поиск по указателям
Greenwood N.N., Earnshaw A. — Chemistry of the Elements
Обсудите книгу на научном форуме Нашли опечатку?
Название: Chemistry of the ElementsАвторы: Greenwood N.N., Earnshaw A. Аннотация: When this innovative textbook first appeared in 1984 it rapidly became a great success throughout the world and has already been translated into several European and Asian languages. Now the authors have completely revised and updated the text, including more than 2000 new literature references to work published since the first edition. No page has been left unaltered but the novel features which proved so attractive have been retained. The book presents a balanced, coherent and comprehensive account of the chemistry of the elements for both undergraduate and postgraduate students. This crucial central area of chemistry is full of ingenious experiments, intriguing compounds and exciting new discoveries. The authors specifically avoid the term `inorganic chemistry' since this evokes an outmoded view of chemistry which is no longer appropriate in the final decade of the 20th century.
Язык: Рубрика: Химия /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Издание: second editionГод издания: 1997Количество страниц: 1340Добавлена в каталог: 18.02.2007Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
728 727 728 408 646 722 727 728 726 728 671 667 758 759 728—729 728—729 725 726 726—727 727 726 727 728 408 646 722—725 722 725 730 734 736 856 857 729 894—894 897 898 64 894—896 894 895 896 898 900 901 11 see “Sodium 926 655 Absolute configuration, determination of 1125 1126 Abundance of elements, Tables of 1294 Acetylides see “Carbides” Acid strength of binary hydrides 49 51 Acid strength of binary hydrides of HF 815 Acid strength of binary hydrides of oxoacids (Pauling's rales) 50 Acidity function 51 52 Actinide contraction 1264 Actinide elements, abundance 1253 Actinide elements, alkyls and aryls 1278 Actinide elements, atomic and physical properties 1263 Actinide elements, carbonyls 1278 Actinide elements, chalcogenides 1471 Actinide elements, complexes, +1 oxidation state 1278 Actinide elements, complexes, +2 oxidation state 1278 Actinide elements, complexes, +3 oxidation state 1277 Actinide elements, complexes, +4 oxidation state 1275 Actinide elements, complexes, +5 oxidation state 1274 Actinide elements, complexes, +6 oxidation state 1273 Actinide elements, complexes, +7 oxidation state 1273 Actinide elements, coordination numbers and stereochemistries 1267 Actinide elements, cyclopentadienyls 1278 Actinide elements, discovery 1252 Actinide elements, group trends 1264—1267 Actinide elements, halides 1269—1272 Actinide elements, hydrides 64 1267 Actinide elements, lanthanide-like behaviour 1251 1264 1266 Actinide elements, magnetic properties 1272 1273 Actinide elements, mixed metal oxides 1269 Actinide elements, organometallic compounds 1278—1280 Actinide elements, oxidation states 1268 Actinide elements, oxides 1268—1269 Actinide elements, preparation of artificial elements 1252—1262 Actinide elements, problems of isolation and characterization 1251 1260 1262 1264 Actinide elements, redox behaviour 1265—1266 Actinide elements, separation from used nuclear fuels 1260—1262 Actinide elements, sepectroscopic properties 1272—1273 Actinium see “Actinide elements” “Group Actinium, abundance 945 Actinium, discovery 944 Actinium, radioactive decay series 1254 Actinoid see “Actinide elements” Actinon see “Actinide elements” Actinylions 1273 1274 Activated carbon 274 see Adenine 61 62 Adenosine triphosphate (ATP) in life processes 528 1101 Adenosine triphosphate (ATP) in nitrogen fixation 1035 1036 Adenosine triphosphate (ATP) in phosphorus cycle 476 Adenosine triphosphate (ATP) in photosynthesis 125 Adenosine triphosphate (ATP), discovery in muscle fibre 474 Agate 342 Air 411 604 889 890 Alane 227 Albite 357 Alkali metals (Li, Na, K. Rb, Cs, Fr) see individual metals: Li Na K Rb Cs Fr Alkali metals (Li, Na, K. Rb, Cs, Fr), abundance 69 Alkali metals (Li, Na, K. Rb, Cs, Fr), alkoxides 87 Alkali metals (Li, Na, K. Rb, Cs, Fr), atomic properties 75 Alkali metals (Li, Na, K. Rb, Cs, Fr), biological systems containing 70 71 73 97 Alkali metals (Li, Na, K. Rb, Cs, Fr), carbonates 59—90 Alkali metals (Li, Na, K. Rb, Cs, Fr), chelated complexes of 112 Alkali metals (Li, Na, K. Rb, Cs, Fr), chemical reactivity of 76 Alkali metals (Li, Na, K. Rb, Cs, Fr), complexes of 90 Alkali metals (Li, Na, K. Rb, Cs, Fr), crown-ether complexes of 90 Alkali metals (Li, Na, K. Rb, Cs, Fr), cryptates of 90 Alkali metals (Li, Na, K. Rb, Cs, Fr), cyanates 324 Alkali metals (Li, Na, K. Rb, Cs, Fr), cyanides 321 Alkali metals (Li, Na, K. Rb, Cs, Fr), discovery of 68 69 Alkali metals (Li, Na, K. Rb, Cs, Fr), flame colours of 75 Alkali metals (Li, Na, K. Rb, Cs, Fr), halides imides, amides 99—102 Alkali metals (Li, Na, K. Rb, Cs, Fr), hydrides, reactions of 84 Alkali metals (Li, Na, K. Rb, Cs, Fr), hydrogen carbonates (bicarbonates) 88 Alkali metals (Li, Na, K. Rb, Cs, Fr), hydroxides 86 87 Alkali metals (Li, Na, K. Rb, Cs, Fr), imides, amides, bonding in 80 Alkali metals (Li, Na, K. Rb, Cs, Fr), imides, amides, properties of 82—83 Alkali metals (Li, Na, K. Rb, Cs, Fr), intercalation compounds with graphite 293 294 Alkali metals (Li, Na, K. Rb, Cs, Fr), intermetallic compounds with As, Sb, Bi 554 555 Alkali metals (Li, Na, K. Rb, Cs, Fr), isolation of 68 69 Alkali metals (Li, Na, K. Rb, Cs, Fr), nitrates 89 Alkali metals (Li, Na, K. Rb, Cs, Fr), nitrites 90 Alkali metals (Li, Na, K. Rb, Cs, Fr), organometallic compounds 106 Alkali metals (Li, Na, K. Rb, Cs, Fr), oxides 84 Alkali metals (Li, Na, K. Rb, Cs, Fr), oxoacid salts 87—90 Alkali metals (Li, Na, K. Rb, Cs, Fr), ozonides 85 Alkali metals (Li, Na, K. Rb, Cs, Fr), peroxides 84 85 Alkali metals (Li, Na, K. Rb, Cs, Fr), physical properties 74 75 Alkali metals (Li, Na, K. Rb, Cs, Fr), polysulfides 677—679 679 681 Alkali metals (Li, Na, K. Rb, Cs, Fr), reactions in liquid ammonia 78—79 Alkali metals (Li, Na, K. Rb, Cs, Fr), sesquioxides 85 Alkali metals (Li, Na, K. Rb, Cs, Fr), solubilites in liquid ammonia 78 Alkali metals (Li, Na, K. Rb, Cs, Fr), solutions in amines 79 Alkali metals (Li, Na, K. Rb, Cs, Fr), solutions in liquid ammonia 77—79 Alkali metals (Li, Na, K. Rb, Cs, Fr), solutions in polyetbers 79 Alkali metals (Li, Na, K. Rb, Cs, Fr), suboxides 84 85 86 Alkali metals (Li, Na, K. Rb, Cs, Fr), sulfides 679 681 682 Alkali metals (Li, Na, K. Rb, Cs, Fr), superoxides 84 85 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), abundance 108—110 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), atomic properties 111 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), chemical reactivity of 112 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), complexes of 122—127 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), halides, crystal structures of 117—118 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), halides, uses 118 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), hydride halides, MHX 119 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), hydrides 64 115 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), hydroxides 119—122 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), intermetallic compounds with As, Sb, Bi 554 555 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), organometallic compounds 127—138 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), oxides 119 121 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), oxoacid salts 122 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), ozonides 119 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), peroxides 119 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), physical properties 112 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), stereochemistry of 114 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), sulfides 679 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), superoxides 121 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), thermal stability of oxoacid salts 113 Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), univalent compounds of 113 see Mg Ca Sr Ba Ra” Alkene insertion reactions (Ziegler) 259 260 Alkene metathesis 1038 Alkene metathesis, polymerization (Ziegler — Natta) 240 241 972 Allotelluric acid 782 Allotropy see individual elements: B C P S Alnico steel 1114 1146 Aluminium see “Group 13 elements” Aluminium hydroxide, bayerile and gibbsite 243 245 Aluminium ion, hydration number of 604 Aluminium oxide hydroxide, diaspore and boehmite 243 Aluminium oxides see ”Portland cement” “High-alumina Aluminium oxides, "Saffil" fibres 244 Aluminium oxides, catalytic activity 244 Aluminium oxides, corundum 242 243 Aluminium oxides, structural classification 242 243 Aluminium trimethyl, 291 258 259 Aluminium trimethyl, 131 Aluminium, abundance 217 Aluminium, alloys 220 Aluminium, borohydride 169 228 Aluminium, chalcogenides 252 Aluminium, halide complexes 233—237 Aluminium, heterocyclic organo-AIN oligomers 265 266 Aluminium, history of 216 Aluminium, hydrides 227 Aluminium, hydrido complexes 228—231 Aluminium, III-V compounds 255 258 Aluminium, lower haiides AIX, AIX2 233 Aluminium, organometallic compounds 257—266 Aluminium, orthophosphale AIPO4 526 Aluminium, production 216. 218—226 Aluminium, ternary oxide phases 247—252 Aluminium, trialkyls and triaryls 257 258—260 Aluminium, trialkyls and triaryls in Ziegler — Natta catalysis 259—263 972 Aluminium, trialkyls and triaryls, dimeric structure of 253 Aluminium, trialkyls and triaryls, preparation 259 Aluminium, trichloride 233 Aluminium, trichloride adducts, structure of 234 235 672 Aluminium, trichloride, Friedel — Crafts activity of 234 236 Aluminium, trichloride, structure 234 Aluminium, trihalide complexes 233—237 Aluminium, uses 220 Alumino-silicates see “Silicate minerals” Alumino-thermic process 1003 Alums 216 Alums, chrome 1027 Alums, chromeiron 1088 Alums, chromerhodium 1129 Alums, chrometitanium 970 Alums, chromevanadium 993 Amalgamation process 1175 1201 1203 Americium 1252 1262 see Amethyst 342 Amidopolyphosphates 506 Aminoboranes 209 Ammonia, adduct with NI3 441 Ammonia, chemical reactions 423 424 486 Ammonia, fertilizer applications 422 Ammonia, hydrazine, production from (Raschig synthesis) 427 428 Ammonia, industrial production 408 419 Ammonia, inversion frequency 423 Ammonia, inversion of, discovery 403 Ammonia, liquid, acid-base reactions in 425 Ammonia, liquid, alkali metal solutions in 77—79 392 Ammonia, liquid, ammonolysis of 535 Ammonia, liquid, amphoteric behaviour in 425 Ammonia, liquid, discovery of coloured metal solutions 408 Ammonia, liquid, H bonding in 52—55 422 Ammonia, liquid, metathesis reactions in 425 Ammonia, liquid, redox reactions in 425 Ammonia, liquid, solubility of compounds in 424 425 Ammonia, liquid, solvate formation in 425 Ammonia, liquid, solvent properties of 77—79 422 424—426 Ammonia, liquid, syntheses in 426 and passim Ammonia, liquid, synthesis of metal cluster ions in 392 393 Ammonia, nitric acid production from 466 467 Ammonia, odour 420 Ammonia, physical properties 422 423 Ammonia, production statistics 420 Ammonia, synthesis of (Haber — Bosch) 408 420 Ammonia, uses of 422 Ammonium nitrate, explosive decomposition of 466 469 Ammonium nitrate, thermolysis of 443 469 Ammonium nitrite, decomposition to 409 Ammonium phosphates 524 Ammonium salts 422 Amosites 351 Amphiboles 351 Amphoteric behaviour of 697 Amphoteric behaviour of 811 Amphoteric behaviour of 981 Amphoteric behaviour of Al and Ga 225 Amphoteric behaviour of ZnO 1209 Amphoteric behaviour, definition 225 Amphoteric cations 52 Anderson structure 1011 Andrieux's phosphide synthesis 489 Andrussow process for HCN 321 Angeli's salt 459 460 Angular functions 1285 1288 1289 Anorthite 357 358 Anti-knock additives 371 799 Anti-tumour activity of 1163 Antiferroelectrics 58 Antimonides 554 Antimonides, monious acid, 575 578 Antimonite esters 561 Antimony, abundance and distribution 548—549 Antimony, allotropes 551—552 Antimony, alloys 549 554 Antimony, aniino derivatives 561 Antimony, atomic properties 550 Antimony, catenation 583 Antimony, chalcogenides 581 582 Antimony, chemical reactivity 552 553 577 Antimony, cluster anions 553 588 Antimony, coordination geometries 554 Antimony, encapsulated 554 Antimony, halide complexes 564—570 Antimony, halides 558—566 see trihalides” “Antimony pentahalides” “Antimony oxide-halides” “Antimony mixed “Antimony halide Antimony, halogeno-organic compounds” 596 598 Antimony, history 547 Antimony, hydride 551—558 Antimony, intennetallic compounds see “Antimonides” Antimony, metal-metal bonded clusters 583 588 589 Antimony, mixed halides 563 562 Antimony, organometaHic halides 596 597 Antimony, organometallic compounds 592 596—598 Antimony, oxidation state diagram 578 Antimony, oxide 572—574 Antimony, oxide halides 570—572 Antimony, oxides and oxocompounds 572—578 Antimony, oxoacid salts of 591 Antimony, pentahalides 561—562 568—570 785 Antimony, pentaphenyl 545 Antimony, physical properties 551. 552 Antimony, selenium complex anions 581 Antimony, sulfide, 547 549 580—581 648 Antimony, trichloride solvent system 560 Antimony, trifluoride as fluorinating agent 560 Antimony, trihalides 558—561 564—566 569 Antimony, uses 549 Antimony, volt-equivalent diagram 578 Antintonates 577 Apatites 109 475 480 525 Apatites, reduction to phosphides 489 Aquaregia 790 792 1179 Aragonite 109 Arenes as 940 Argentite (silver glance) 1174 Argon see “Noble gases” Argon, atomic and physical properties 891 Argon, clathrates 893 Argon, discovery 889
Реклама
 |
|
О проекте
|
|
О проекте



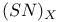 polymer, partially halogenated derivatives
polymer, partially halogenated derivatives 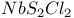 structure
structure 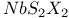
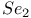 as ligand
as ligand 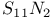
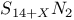
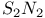
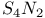
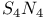
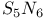
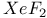
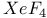
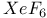
 -Process in stars
-Process in stars  -alumina
-alumina  -monoclinic sulfur
-monoclinic sulfur 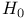 (Hammett)
(Hammett) 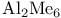
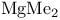
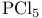
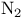
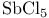
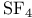 (Lewis acid-base)
(Lewis acid-base) 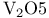
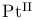 complexes
complexes 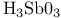
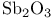
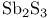
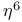 ligands
ligands