Авторизация
Поиск по указателям
Greenwood N.N., Earnshaw A. — Chemistry of the Elements
Обсудите книгу на научном форуме Нашли опечатку?
Название: Chemistry of the ElementsАвторы: Greenwood N.N., Earnshaw A. Аннотация: When this innovative textbook first appeared in 1984 it rapidly became a great success throughout the world and has already been translated into several European and Asian languages. Now the authors have completely revised and updated the text, including more than 2000 new literature references to work published since the first edition. No page has been left unaltered but the novel features which proved so attractive have been retained. The book presents a balanced, coherent and comprehensive account of the chemistry of the elements for both undergraduate and postgraduate students. This crucial central area of chemistry is full of ingenious experiments, intriguing compounds and exciting new discoveries. The authors specifically avoid the term `inorganic chemistry' since this evokes an outmoded view of chemistry which is no longer appropriate in the final decade of the 20th century.
Язык: Рубрика: Химия /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Издание: second editionГод издания: 1997Количество страниц: 1340Добавлена в каталог: 18.02.2007Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
Terbium 1229 (see also “Lanthanide elements”) Terbium, +4 oxidation state 1237 1239 Tetracyanoethylene complexes, bonding in 931 Tetrafluoroethylene complexes, bonding in 932 Tetrafluoronitronium cation 439 Tetrahalogenophosphonium cations 499—500 Tetrahedral complexes 914 Tetrahydroaluminate ion, as ligand 231 Tetrahydroborates of Al 260 228 229 Tetrahydroborates of Ga 231 Tetrahydroborates of Zr and Hf 969 Tetrahydroborates, use in synthesis 166—168 Tetrametaphosphimate conformers 542 Tetrathionates, 717 718 Thallium see also “Group 13 elements” Thallium, abundance 217 Thallium, chalcogenides 252—254 Thallium, discovery 217 Thallium, halide complexes 240 Thallium, III—V compounds 255—258 Thallium, lower halides 241 Thallium, monohalides 241 242 Thallium, organometallic compounds 261 265 Thallium, oxides 246 Thallium, production 221 Thallium, similarity of 226 Thallium, trihalides 239 Thallium, triiodide 239 240 Thiazyl halides NSX 736—738 Thio-urea 317 Thioarsenites 580 Thiocarbonyl (CS) complexes 319 Thiocyanates 320 324 Thiocyanates as ambidentate ligands 326—327 907 920 Thioethers as ligands 673 Thionitrosyl (NS) complexes 453 454 Thionyl bromide 694 Thionyl chloride 693 694 Thionyl chloride, relation to 694 Thionyl fluoride 688 693 694 Thionyl fluoride, mixed fluoride chloride 694 Thiophosphoryl, halides, 500 502 Thiophosphoryl, pseudohalides 501 Thioselenates 783 Thiosulfates, 705 714 715 Thiosulfates, 714 715 Thiosulfates, 714 715 Thiosulfates, 714 715 Thiosulfates, 714 1186 1187 Thiosulfuric acid, 705 714 Thiosulfuric acid, 714 Thiosulfuric acid, 714 Thixotropy 356 968 Thorium see also “Actinide elements” Thorium, abundance 1253 Thorium, bis(cyclooctatetraene) 942 Thorium, production and uses 1255 Thorium, radioactive decay series 1254 Thorium, redox behaviour 1265—1267 Thorium, use as a nuclear fuel 1258 1259 Thortveitite 348 Three-centre bonds in Al trialkyls and triaryls 258 Three-centre bonds in beryllium alkyls 127 Three-centre bonds in magnesium alkyls 127 Three-centre bonds, 37 Three-centre bonds, BBB bond 158 Three-centre bonds, BHB bond 64 151ff Three-centre bonds, BHM bond 177 Thulium 1228 (see also “Lanthanide elements”) Thymine 61 62 Thyroxine 794 795 Tin in antiquity 367 368 Tin, abundance 368 Tin, allotropes 373 Tin, alloys 370 Tin, atomic properties 371—372 Tin, bis(cyclopentadienyl) 402 Tin, chalcogenides 389 Tin, chemical reactivity and group trends 373 Tin, cluster anions 374 393 Tin, cluster complexes 383 395 Tin, compounds, use of 385 400 Tin, dibromide 380 Tin, dichloride 379 380 Tin, difluoride 379 Tin, dihalides 375 377—381 Tin, diiodide 380 Tin, dioxide 384 386 387 388 400 Tin, halogeno complexes 377—381 399 Tin, hydrides 375 Tin, hydroxo species 383 395 Tin, isolation and purification 369 Tin, metal-metal bonded compounds 391 396 399—404 Tin, monomeric 391 Tin, monoxide 377 383 387 388 Tin, nitrates 387 Tin, organometallic compounds 396—403 Tin, organometallic compounds, oligomerization 396 Tin, organometallic compounds, production statistics 400 Tin, organometallic compounds, toxic action 400 Tin, organometallic compounds, uses of 400 Tin, oxoacid salts 387 388 Tin, physical properties 371 373 Tin, production statistics 368 379 Tin, pseudohalogen derivatives 389 Tin, sulfide 389 Tin, tetrahalides 375 381 385 Tin, uses 370 385 Titanates 963—964 Titanates, ferroelectric properties 963 Titanium see also “Group 4 elements” Titanium, abundance 955 Titanium, alkoxides 967 Titanium, alkyls and aryls 973 Titanium, alum 970 Titanium, bronzes 964 Titanium, carbonyls 973 Titanium, complexes, +3 oxidation state 969—971 Titanium, complexes, +4 oxidation state 967—969 Titanium, complexes, lower oxidation states 971—975 Titanium, complexes, with S 670 672 Titanium, compounds with oxoanions 966 Titanium, cyclooctatetraene complex 943 Titanium, cyclopentadienyls 973—975 Titanium, dioxide 959 Titanium, discovery 954 Titanium, estimation using 968 Titanium, halides 964—966 Titanium, mixed metal oxides (titanates) 963—964 Titanium, nonstoichiometric oxide phases 642 961 Titanium, organometallic compounds 972—975 Titanium, production and uses 955—956 Titanium, sulfides 962 Titanium, “sponge” 956 Tobermorite gel 252 Tolman's cone angle 494 Tooth enamel 477 Toothpastes, calcium compounds in 528 Trans-effect 1164 Trans-effect in 1163 1164 Trans-effect in 1127 Trans-effect in 1085 Trans-influence 1164 1165 Trans-influence in 1165 Trans-influence in 1085 Transactinide elements 1280—1284 Transferrin 1103 Transistor action 331 332 Transistor action, chemistry of manufacture 332 Transistor action, discovery 331 Transition element ions, coordination chemistry 905—943 Transition elements see also “Transition element ions” Transition elements, characteristic properties 905 Transition elements, definition of 905 Transuranium elements see also “Actinide elements” Transuranium elements, discovery of 21 29 1252 Transuranium elements, extraction from reactor wastes 1262 Tri-capped trigonal prismatic complexes 917 Tricalcium aluminate, 251 (see also “Portland cement”) Tricalcium phosphate $[Ca_5(PO_4)_3OH] 524 Tridymite 343 Trigonal bipyramidal complexes 914 Trigonal prismatic complexes 915 Triperiodic acid see “Periodic acids” Triphosphoric acid 512 Tris(dimethylamino)phosphine 533 Trithiocarbonates 317 Trithionates, 717 718 Tritium, atomic properties 34 Tritium, discovery 33 Tritium, physical properties 35 Tritium, preparation of tritiated compounds 42 Tritium, radioactivity of 42 Tritium, synthesis 41 Tritium, uses as a tracer 42 Tropylium (cycloheptatrienyl) 942 Tungstates 1009—1016 Tungsten see also “Group 6 elements” Tungsten, abundance 1003 Tungsten, benzene tricarbonyl 941 Tungsten, blues 1008 Tungsten, bronzes 1016 Tungsten, carbonyls 928 1037—1038 Tungsten, carbyne complexes 929 Tungsten, chalcogenides 1017—1018 Tungsten, complexes, +2 oxidation state 1031—1034 Tungsten, complexes, +3 oxidation state 1027—1031 Tungsten, complexes, +4 oxidation state 1025—1027 Tungsten, complexes, +5 oxidation state 1024 1025 Tungsten, complexes, +6 oxidation state 1023—1024 Tungsten, complexes, with S 670 Tungsten, cyclopentadienyl derivatives 1039 Tungsten, discovery 1002 Tungsten, halides and oxohalides 1019—1023 Tungsten, heteropolyacids and salts 1014—1016 Tungsten, hexacarbonyl 928 1038 Tungsten, isopolyacids and salts 1009—1014 Tungsten, nonstoichiometric oxides 1008 Tungsten, organometallic compounds 829 940 941 1037—1039 Tungsten, oxides 1007—1009 Tungsten, production and uses 1003 Tungstic acid 1010 Tungstocene 1038 Turnbull's blue 1094 Tutton salts of copper 1190 Tutton salts of vanadium 993 Type metal 547 549 Tyrian purple 790 791 793 u (ungerade), definition of 938 Ultramarines 354 359 Units, conversion factors 1293 Units, non-SI 1293 Universe, expansion of 2 5 Universe, origin of 1 2 Uranium see also “Actinide elements” Uranium hexafluoride 1259 1269—1271 Uranium oxides, nonstoichiometry in 643 Uranium, abundance 1253 Uranium, bis(cyclooctatetraene) 942 Uranium, isotopic enrichment 1259 Uranium, production 1255 Uranium, radioactive decay series 1254 Uranium, redox behaviour 1265—1267 Uranium, variable atomic weight 17 Uranyl ion 1266 1269 1273—1274 Urea 305 311 323 422 Urea, hydrazine production from 429 Urea, phosphate 524 Urea, Wohler's synthesis 408 Valence bond theory of transition metal complexes 921-924 Valence, periodic trends in 27 Valinomycin 96 Van Arkel-de Boer process 956 Vanadates 981 983—987 Vanadium see also “Group 5 elements” Vanadium, abundance 977 Vanadium, accumulation in blood of invertebrates 999 Vanadium, alkyls and aryls 999 Vanadium, biochemistry of 999 Vanadium, bronzes 987 Vanadium, carbonyl 928 1000 Vanadium, chalcogenides 988 Vanadium, complexes, +2 oxidation state 998 Vanadium, complexes, +3 oxidation state 996—998 Vanadium, complexes, +4 oxidation state 994—996 Vanadium, complexes, +5 oxidation state 994 Vanadium, compounds with oxoanions 993 Vanadium, cyclopentadienyls 939 1000 Vanadium, discovery 976 Vanadium, dithiolene complexes 674 675 Vanadium, halides and oxohalides 988—993 Vanadium, hexacarbonyl 828 928 Vanadium, isopolyacids and salts 983—987 Vanadium, nonstoichiometric oxides 982 Vanadium, organometallic compounds 927 939 941 942 997—1001 Vanadium, oxides 981—983 Vanadium, production and uses 977 Vanadocene 1000 Vanadyl compounds 982 995 996 Vaska's compound 615 616 1135—1137 Venus, atmosphere of 645 646 Vermiculite 349 357 Viscose rayon 317 Vitamin 1138 1139 1226 Volt equivalent, definition 434 Volt equivalent, diagrams 436—438 Vortmann's sulfate 1127 Vulcanization of rubber 646 Wackenroder's solution 717 719 Wacker process 1172 Wade's rules 161 162 181 553 590 591 Water supplies, treatment of 120 Water, acid-base behaviour 48 628 Water, aquo complexes 625 Water, autoprotolysis constant 48 Water, chemical properties 627 Water, clathrate hydrates 626 627 Water, distribution and availability 621—623 Water, H bonding in 52—55 Water, heavy (D2O) 623 Water, history 620 Water, hydrates 625—627 Water, hydrolysis reactions 627 Water, ice, polymorphism 624 Water, ionic product of 48 Water, Karl Fischer reagent for 627 Water, lattice water 625 Water, physical properties 623—625 754 Water, pollution of 622 Water, polywater 632 633 Water, purification and recycling 622 623 Water, self ionic dissociation of 48 Water, tritiated 623 Water, zeolitic water 625 Water-gas shift reaction 38 311 421 1106 Water-gas shift reaction in Haber — Bosch 421 White arsenic see “Arsenic oxide” “ Wilkinson's catalyst 43 1134—1135 Wilson's disease 1198 Wittig reaction 474 475 545
Реклама
 |
|
О проекте
|
|
О проекте



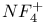
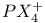
 , preparation and structure
, preparation and structure 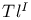 to alkali metals
to alkali metals![$Tl^{I}[I_3]^{—}$](/math_tex/bfbeaa7d90d4fd03dc740157b64ea9bd82.gif)
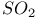 and
and 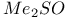 as ionizing solvent
as ionizing solvent 
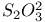


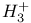 ion
ion 
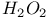
 complexes
complexes  complexes
complexes ![$[OsNCl_5]^{2—}$](/math_tex/304b130c1e16d935570ae87e4cf3952c82.gif)
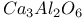 , structure
, structure 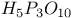
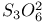 , preparation and structure
, preparation and structure 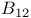
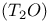
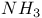 synthesis
synthesis