|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Hermanson G.T. — Bioconjugate Techniques |
|
|
 |
| Предметный указатель |
SPDP, activation of antibodies 509
SPDP, activation of antibody 508
SPDP, activation of liposomes 547
SPDP, activation of PE 544
SPDP, activation of toxins 507
SPDP, alkaline phosphatase, conjugation to DNA 663
SPDP, alkaline phosphatase, modified 664
SPDP, analogs of 65 231
SPDP, cleavability of 293
SPDP, comparison to SMPB 242
SPDP, conjugation of avidin to phycobiliproteins using 588
SPDP, conjugation of proteins to liposomes using 564
SPDP, creation using 2,2'-dipyridyl disulfide 133
SPDP, enzyme activation using 637
SPDP, general description and use 230
SPDP, inactivation of gelonin and PAPs 509
SPDP, liposome conjugation using 542
SPDP, modification of amine-DNA probes 653
SPDP, modification of dextran with 624 628
SPDP, pyridyl disulfide reactions of 151
SPDP, thiolation of antibodies 518
SPDP, use for thiolation 64
Speakman 221
Speck 407
Sphingolipids 536 537 539
Sphingolipids, types 536
Sphingolipids, use in liposomes 537
Sphingomyelin 536 537 550
Sphingosine 534 536 548
Spiegel 116 121 361
Srinivasachar 209 211
Stahlberg 369
Stanley 195
Starch 35
Stark 206
Staros 140 173 176 178 190 193 194 196 254 438
Stearic acid 536
Stearylamine, activation with SPDP 565
Stearylamine, functional derivatives 539
Steer 36
Steric hindrance 306
Sternberger 584
Stewart 179 358 359
Stickel 328
Stilbenedisulfonate receptor 310
Stirpe 498
Stoke’s law 298
Stoke’s shift 344 351 352 587
Stoke’s shift of AMCA derivatives 339
Stoke’s shift of BODIPY 493/503 C3 hydrazide 348
Stoke’s shift of BODIPY FL C3 hydrazide 349
Stoke’s shift of BODIPY FL C3-SE 343
Stoke’s shift of BODIPY fluorophores 342
Stoke’s shift of Lucifer Yellow fluorophores 359
Stoke’s shift of phycobiliproteins 362
Stoke’s shift of tandem phycobiliprotein conjugates 364
Stoke’s shift, definition of 298
Straubinger 552
Streefkerk 472
Streptavidin 254 287 298 372 378 382 384 386 388 390 492 646
Streptavidin, activation with bis-hydrazides 222 589
Streptavidin, activation with sulfo-SMCC 576
Streptavidin, alkaline phosphatase conjugate 394 661
Streptavidin, binding to ABNP cross-links 288
Streptavidin, binding to sulfo-SBED cross-links 289
Streptavidin, biotin binding site 372
Streptavidin, comparison to avidin 571
Streptavidin, conjugation to ferritin using glutaraldehyde 583
Streptavidin, conjugation to HRP using reductive amination 581 582
Streptavidin, conjugation to liposomes 554
Streptavidin, conjugation using glutaraldehyde 583
Streptavidin, conjugation using reductive amination 580
Streptavidin, conjugation with enzymes using SMCC 576
Streptavidin, conjugation with phycoerythrin 363 588
Streptavidin, effect of periodate 294
Streptavidin, enzyme-labeled 572 575
Streptavidin, fluorescent labeling of 584
Streptavidin, general description and use 570
Streptavidin, gold-labeled 574 596 602
Streptavidin, hydrazide-activated 222 574 637
Streptavidin, immobilized 291 379 382 387
Streptavidin, interaction with biotin 373
Streptavidin, interaction with biotinylated antibodies 488 492
Streptavidin, interaction with iminobiotin 372 380
Streptavidin, maleimide-activated 578
Streptavidin, modification with AMCA-NHS 587
Streptavidin, modification with FITC 585
Streptavidin, modification with Lissamine rhodamine sulfonyl chloride 586
Streptavidin, optimal fluorescein labeling level 586
Streptavidin, preparation of conjugates 575
Streptavidin, purification using immobilized iminobiotin 381
Streptavidin, rate of biotin interaction with 372
Streptavidin, structure and properties 571
Streptavidin, thiolation, 2-iminothiolane 588
Streptavidin, thiolation, using SATA 62 579
Streptavidin, use in assay systems 572
Streptomyces avidinii 571
Streptomyces pilosus 370
Strickland 631
Strittmatter 532
Stroh 157
Strottmann 304
Stryer 364
Stuchbury 209
Stump 599
Styrene/4-vinylbenzoic acid copolymer 153 183
Subramanian 365
Substrates 630
Subunits, disulfide-linked 9
Succinaldehyde 215
Succinic acid 91 94
Succinic acid in cross-bridge of EGS 199 296
Succinic anhydride 90 145
Succinic anhydride, general description and use 91
Succinic anhydride, modification of amines 93
Succinic anhydride, modification of mPEG with 615
Succinic anhydride, modification of PEG with 609 614
Succinic anhydride, reaction with aminodextran 624
Succinimidyl 4-(((iodoacetyl)amino) methyl)cyclohexane-1-carboxylate see “SIAC”
Succinimidyl 6-((((4-iodoacetyl)amino)methyl)-cyclohexane-1-carbonyl)amino) hexanoate see “SIACX”
Succinimidyl 6-((iodoacetyl)-amino)hexanoate see “SIAX”
Succinimidyl 6-(6-(((4-iodoacetyl) amino)hexanoyl)amino)hexanoate see “SIAXX”
Succinimidyl acetylthiopropionate see “SATP”
Succinimidyl carbonate 142 143 156 202
Succinimidyl carbonate, comparison with CDI coupling 615
Succinimidyl carbonate, formation of 611 612
Succinimidyl carbonate, hydrolysis of 611
Succinimidyl carbonate, reaction with amines 611
Succinimidyl glutarate 609
Succinimidyl p-formylbenzoate see “SFB”
Succinimidyl p-formylphenoxyacetate see “SFPA”
Succinimidyl succinate 609
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate see “SMCC”
Succinimidyl-4-(p-maleimidophenyl)butyrate see “SMPB”
Succinimidyl-7-amino-4-methylcoumarin-3-acetic acid see “AMCA-NHS”
Succinimidyloxycarbonyl-a-methyl-a-(2-pyridyldithio)toluene see “SMPT”
Succinimidyl—6—(biotin-amido)hexanoate see “NHS-LC-biotin”
Succinylation 91 (see also “Succinic anhydride”)
Sucrose 35
Sugars, functional groups 29 (see also “Polysaccharides”)
Sugars, immobilized 517
Sugars, labeling with biocytinhydrazide 393
Sugars, modification with dimines 107
Sugars, reducing 108 141
Sulfenylthiosulfate 131 516
Sulfenylthiosulfate as blocking agent 132
Sulfhydryl oxidation, metal catalyzed 232
Sulfhydryls 107
Sulfhydryls in immobilized reductants 85 87
Sulfhydryls of 3-galactosidase 460 577
| Sulfhydryls of SAMSA-fluorescein 311
Sulfhydryls of SATA 60
Sulfhydryls, acetylated 467
Sulfhydryls, activated with TNB 151
Sulfhydryls, addition to double bonds 149
Sulfhydryls, alkylation by maleimides 148
Sulfhydryls, assay with Ellman’s reagent 88
Sulfhydryls, biotinylation of 384
Sulfhydryls, blocking agents, permanent and reversible 126 129 133
Sulfhydryls, complexation to metals 370
Sulfhydryls, conjugation with haptens containing 439
Sulfhydryls, coupling of radionuclides 365
Sulfhydryls, creation of 231
Sulfhydryls, creation of AMBH 71
Sulfhydryls, creation of cystamine 72
Sulfhydryls, creation of DTT 77
Sulfhydryls, creation of N-acetyl homocysteine thiolactone 68
Sulfhydryls, creation of SAMSA 70
Sulfhydryls, creation of SATA 60
Sulfhydryls, creation of SATP 63
Sulfhydryls, creation of SMPT 66
Sulfhydryls, creation of sodium borohydride 88
Sulfhydryls, creation of SPDP 64
Sulfhydryls, creation of, by reduction of disulfides 76
Sulfhydryls, creation of, by reduction with 2-mercaptoethylamine 82
Sulfhydryls, creation of, by reduction, using TCEP 83
Sulfhydryls, creation of, on antibodies 461
Sulfhydryls, creation of, on antibodies with 2-iminothiolane 465
Sulfhydryls, creation of, using 2-iminothiolane 57
Sulfhydryls, dative binding to gold 594
Sulfhydryls, deprotection of acetylated 578
Sulfhydryls, detection using Ellman’s reagent 87 132
Sulfhydryls, formation of pyridyl disulfide groups 133
Sulfhydryls, introduction of 56
Sulfhydryls, modification with 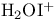 401 401
Sulfhydryls, modification with 2-bromoethylamine 106
Sulfhydryls, modification with ethylenimine 105
Sulfhydryls, permanent and reversible linkages with 208
Sulfhydryls, preventing oxidation to disulfides 57
Sulfhydryls, reaction by disulfide exchange 150
Sulfhydryls, reaction of 11 146
Sulfhydryls, reaction with 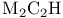 250 250
Sulfhydryls, reaction with alkyl halides 209 226
Sulfhydryls, reaction with aminoethyl-8 104
Sulfhydryls, reaction with anhydrides 146
Sulfhydryls, reaction with APDP 277
Sulfhydryls, reaction with aryl azides 254
Sulfhydryls, reaction with aryl halides 143 149
Sulfhydryls, reaction with ASIB 276
Sulfhydryls, reaction with aziridines 148
Sulfhydryls, reaction with benzophenone-4-iodoacetamide 279
Sulfhydryls, reaction with benzophenone-4-maleimide 280
Sulfhydryls, reaction with biotin-BMCC 384
Sulfhydryls, reaction with BMH 211
Sulfhydryls, reaction with CMC 177
Sulfhydryls, reaction with diazo compounds 153
Sulfhydryls, reaction with difluorbenzene derivatives 212
Sulfhydryls, reaction with EDC 172
Sulfhydryls, reaction with epoxides 142 155 220
Sulfhydryls, reaction with glutaric anhydride 94
Sulfhydryls, reaction with GMBS 245
Sulfhydryls, reaction with haloacetyl compounds 147
Sulfhydryls, reaction with iodoacetate 98
Sulfhydryls, reaction with iodoacetyl-LC-biotin 388
Sulfhydryls, reaction with isothiocyanates 138
Sulfhydryls, reaction with maleic anhydride 129
Sulfhydryls, reaction with maleimides 95 209
Sulfhydryls, reaction with MBS 237
Sulfhydryls, reaction with MPBH 249
Sulfhydryls, reaction with NEM 130
Sulfhydryls, reaction with NHS esters 139 191
Sulfhydryls, reaction with NPIA 248
Sulfhydryls, reaction with PDPH 252
Sulfhydryls, reaction with pyridyl disulfides 151
Sulfhydryls, reaction with SIAB 239
Sulfhydryls, reaction with SIAC 247
Sulfhydryls, reaction with SIAX 245
Sulfhydryls, reaction with SMCC 235
Sulfhydryls, reaction with SMPB 242
Sulfhydryls, reaction with SMPT 233
Sulfide 632
Sulfo-BSOCOES, general description and use 199
Sulfo-DST, general description and use 196
Sulfo-EGS, general description and use 201
Sulfo-GMBS, general description and use 245
Sulfo-GMBS, low immunogenicity of 441
Sulfo-HSAB, general description and use 260
Sulfo-HSAB, similarity to sulfo-SAPB 267
Sulfo-LC-SMPT 66 233
Sulfo-LC-SPDP 65 231 232
Sulfo-LC-SPDP in the conjugation of phycobiliproteins 364
Sulfo-LC-SPDP, activation of liposomes 548
Sulfo-LC-SPDP, use in protein-liposome conjugation 565
Sulfo-MBS, general description and use 239
Sulfo-NHS acetate 127
Sulfo-NHS esters 101 127 190 235 438
Sulfo-NHS esters of  196 196
Sulfo-NHS esters of DTSSP 193
Sulfo-NHS esters of SAED 268
Sulfo-NHS esters of SAND 263
Sulfo-NHS esters of SASD 256
Sulfo-NHS esters of sulfo-DST 196
Sulfo-NHS esters of sulfo-HSAB 260
Sulfo-NHS esters of sulfo-SADP 266
Sulfo-NHS esters of sulfo-SAMCA 272
Sulfo-NHS esters of sulfo-SAPB 266
Sulfo-NHS esters of sulfo-SBED 289
Sulfo-NHS esters of sulfo-SHPP 414
Sulfo-NHS esters of sulfo-SMCC 236
Sulfo-NHS esters, chemistry of 139
Sulfo-NHS, produced using the EDC/sulfo-NHS reaction 140 173 430 439
Sulfo-NHS, use with EDC to activate protein carboxylates 436
Sulfo-NHS-ASA, general description and use 256
Sulfo-NHS-biotin, general description and use 376
Sulfo-NHS-biotin, use in protein biotinylation 377
Sulfo-NHS-LC-ASA, general description and use 256
Sulfo-NHS-LC-biotin, general description and use 379
Sulfo-NHS-SS-biotin, general description and use 382
Sulfo-SADP, general description and use 266
Sulfo-SAMCA, general description and use 270
Sulfo-SANPAH 264
Sulfo-SANPAH, general description and use 263
Sulfo-SAPB, general description and use 266
Sulfo-SBED 291 375
Sulfo-SBED, functional arms of 291
Sulfo-SBED, general description and use 289
Sulfo-SHPP, general description and use 414
Sulfo-SIAB, general description and use 240
Sulfo-SMCC 236 441 462 520
Sulfo-SMCC in hapten-carrier conjugation 439
Sulfo-SMCC use with poly-L-lysine 428
Sulfo-SMCC, activation of antibodies using 521
Sulfo-SMCC, activation of avidin and streptavidin with 576
Sulfo-SMCC, activation of carrier proteins 444
Sulfo-SMCC, activation of enzymes with 461 576 636
Sulfo-SMCC, activation of HRP with 579
Sulfo-SMCC, general description and use 236
Sulfo-SMCC, use in antibody-enzyme conjugation 461
Sulfo-SMPB, general description and use 243
Sulfonamide linkage 141
Sulfonate 127 149 190 196 209 236 239 240 243 245 256 260 263 266 267
Sulfone 213 313
Sulfone in cleavable reagents 293
Sulfone, base labile group in cross-linkers 296
Sulfone, cleavability 198
Sulfonic acid 140
Sulfonyl chloride, chemistry of 140
Sulfonyl of Lissamine rhodamine sulfonyl chloride 323
Sulfonyl of Texas Red sulfonyl chloride 325
Sulfonylhydrazine, of rhodamine derivatives 328
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте