|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Rappoport Z. (ed.) — The Chemistry of Dines and Polyenes (vol. 1) |
|
|
 |
| Предметный указатель |
Enol ethers, radical reactions of 637
Enolic dienes, analysis of 499 500
Enprostil, tritium-labelled, synthesis of 812—815
Ensley, H.E. 915(159) 925
Enthalpies of combustion, of cumulated tetraenes 74
Enthalpies of combustion, of cyclohexadienes 81
Enthalpies of conjugation, of substituted butadienes 77
Enthalpies of formation 69
Enthalpies of formation of  -dicarbonyls 78 -dicarbonyls 78
Enthalpies of formation of allenes 72 73
Enthalpies of formation of annulenes 101—103
Enthalpies of formation of barrelene 86
Enthalpies of formation of bis-allenes 75
Enthalpies of formation of bismethylenecycloalkanes 83—85
Enthalpies of formation of conjugated dienes, acyclic 77—79
Enthalpies of formation of conjugated polyenes, acyclic 87—89
Enthalpies of formation of conjugated polyenes, cyclic 89—91
Enthalpies of formation of cumulated trienes 73 74
Enthalpies of formation of cycloheptadienes 82
Enthalpies of formation of cyclohexadienes 39 81 82
Enthalpies of formation of cyclooctadienes 83
Enthalpies of formation of cyclopentadienes 80 81
Enthalpies of formation of fulvenes 94—98
Enthalpies of formation of isotoluenes 98 99
Enthalpies of formation of nonconjugated dienes, acyclic 71
Enthalpies of formation of nonconjugated dienes, bicyclic 85 86
Enthalpies of formation of triquinacene 86
Enthalpies of formation of xylylenes 99 100
Enthalpies of fusion 70
Enthalpies of hydrogenation 70
Enthalpies of hydrogenation of acyclic polymeric polyenes 72
Enthalpies of hydrogenation of benzocyclobutene 99
Enthalpies of hydrogenation of bismethylenecycloalkanes 84
Enthalpies of hydrogenation of cumulated tetraenes 74 76
Enthalpies of hydrogenation of cyclohexadienes 81
Enthalpies of hydrogenation of cyclooctadienes 83
Enthalpies of hydrogenation of heptafulvenes 97
Enthalpies of hydrogenation of hexatrienes 88
Enthalpies of polymerization, of acyclic polymeric polyenes 72
Enthalpies of sublimation 70
Enthalpies of sublimation of hexatrienes 89
Enthalpies of vaporization 70
Enthalpies of vaporization of acyclic polymeric polyenes 72
Enthalpies of vaporization of cyclohexadienes 81
Enthalpies of vaporization of hexatrienes 88
Enynes, reduction of 453—455
Enzell, C.R. 493(20) 505
Epa, W.R. 439(236b) 477
Epling, G.A. 281(43) 321
Epoxidation 891
Epoxidation stereoselective 665
Epperly, M.W. 844 845(177) 865
Erb, J.M. 831(149) 865
Erden, I. 184(138) 257 914(151) 925
Erickson, K.L. 274(26) 320
Eriksen, J. 919(182) 926
Ermann, P. 459 462(299b) 479
Ermer, O. 40(80) 48(122) 63 64 252(308) 261 559(51) 571(90) 613 614
Ermolaeva, L.V. 179 180(40) 255
Ernest, I. 411(155d) 473
Ernst, L. 590(126) 615
Eros, D. 518(47) 544
Ervin, K.M. 735 738 739(8) 750
Erwin, W.R. 821(112) 864
EsaM, T. 440 441(244b) 477
Eschenmoser, A. 276(31a 31b) 320 374(49) 469 532(99) 546
Escher, S.D. 375(56b) 469
Essenfeld, A.P. 419(165e) 474 514(34) 544
Estok, G.K. 498(39) 505
Eswarakrishnan, V. 374(54b) 469
Etamad, S. 243 246(300) 261
Etemad, S. 158(44) 170
Ethyleries (see also “t-Butylethylene” “1
Ethyleries, acidity of 735 736
Ethyleries, aerobic oxidation of 653
Ethyleries, deprotonation energy of 737 738
Ethyleries, geometry of 737
Eucarvone, photocyclization of 268 269
Eudesmane, photostationary equilibrium with germacrane 272
Eugster, C.H. 169(126) 172 419(165d) 474
Evans, DA. 440 443(245b) 477 812(94) 863
Evans, E.A. 822(115) 864
Evans, J.F. 410(155a) 473
Evans, M.G. 347(112) 356
Evans, W.H. 69(3) 70(9) 104
Evanseck, J.D. 2 17(3) 20 558(46) 597(159) 612 616
Evens, R. 485(10) 504
Evstigneeva, R.P. 813(98) 863
Exchange integrals 228
Excitation energies, adiabatic 13
Excitation energies, vertical 12
Excited states 10—15
Exner, O. 549(18) 611
Eyring, H. 112 117(lc) 119(18) 120 133(lc) 146
Facleau, T.J. 791(42) 862
Fagan, P.J. 942(33) 975
Fallahpour, R.-A. 570 573(88) 614
Fallis, A.G. 180(53) 255 402(133a) 472 511(22) 544
Fan, S.Y. 904(82) 924
Fang, H.L.-B. 157(41) 170
Fang, J.-M. 627 631(32) 650
Fang, W. 71(12) 72(14) 76 77(12) 86(50 51) 88(12) 91(69) 104 105 108 109
Fang, Y.B. 844(179) 865
Farge, G. 394(105e) 471
Farges, G. 43(97) 63
Farid, R. 769(19) 774
Farnesoic acid, tritium-labelled, synthesis of 810—813
Farnesyl diazomethyl ketone, tritium-labelled, synthesis of 810—813
Farquharson, S. 338(65) 355
Farvey, D.H. 465(307) 480
Fattuoni, C 627 635(36b) 650
Fauler, J. 782(20) 861
Faulques, E. 166(116) 169(128) 172
Fayos, J. 183 184(128) 257
Fazlitdinova, N.B. 860(239) 867
Fazon, S. 667(47) 680
Fecapentaene,  - and - and  -labelled, synthesis of 822 823 -labelled, synthesis of 822 823
Feldhuis, M. 638 639(70) 651
Feldman, K.S. 313(110a 110b 111) 324
Felkin, H. 17 19 20(115) 23
Fenske, RF. 207(176) 258
Fenton, D.N. 871 873(10) 886
Ferguson, G. 657(13a 13b) 679
Ferguson, M.D. 315(112a 120) 317(120) 324
Feringa, B.L. 593(144) 615
Ferrar, W.T. 343(92) 356
Ferretti, L. 38(60) 63
Ferro, M.P. 394 396(106a) 471
Ferromagnets, organic 930
Fessenden, R.W. 330 331(16) 341(85) 354 356
Fessner, W.-D. 182 184(108) 185(160) 252(309) 256 258 261 466(312a 312b) 480 575(92a 92b) 614
Fex, T. 405(141a) 472
Fiandanese, V. 452(273) 457(289) 479
Fiddler, S. 415 421 460(171b) 474
Fiecchi, A. 893(15) 922
Field, Mi 17(95) 22
Fielder, S. 791(43 44) 862
Fields, E.K. 920(200) 926
Fieser, L.F. 483 500(5b) 504
Fieser, M. 483 500(5b) 504
Figeys, H.P. 372(47b) 469
Fiksdahl, A. 504(53) 505
Filtgerald, G.R. 236 249(245) 260
Fincher, C.R. 31(21) 62
Findlay, R.H. 179(43) 255
Fink, M.O. 633 640(62) 651
Finnerty, MA. 86 88(56) 108
Finter, J. 343(93) 356
Finzi, C 647(90) 652
Firestone, M.A. 561(63b) 613
| Firestone, R.A. 561(65) 613 849(200) 866
Firestone, R.B. 822(120) 864
Firl, J. 718(60) 719(61) 724(71) 732
Fischer, H.G. 921(210) 926
Fischl, A. 394 396(106a) 471
Fischli, A. 394 396(106d) 471
Fish, R.H. 627 631 632 634 635(38) 650
Fisher, JJ. 35(44) 62 161(72) 171
Fisher, P.V. 289(62) 290(63—66) 322
Fisher, R.W. 902(73) 924
Fitchen, D.B. 166(104) 169(127) 172
Fitzi, K. 530(89) 545
Fitzpatrick, F.A. 783(26) 834(158) 862 865
Fitzpatrick, G.J. 871(17) 886
Fitzsimmons, B. 825(127) 864
Fitzsimmons, B.J. 410(155a) 473
FK-506, 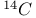 - labelled, synthesis of 840 841 - labelled, synthesis of 840 841
Flagan, R.C. 922(218) 926
Fleming, I. 361 364(8) 395(110) 467 471 673(57c) 681
Fleming, M.P. 428(187a) 475
Flicker, W.M. 11(63) 22
Flippen-Anderson, J.L. 565(70) 613
Florke, U. 33(35) 62
Flory, P.J. 347(114) 356
Floyd, L.J. 838(165) 865
Fluorescence 11
Fluorescence spectroscopy 151
Fluorescence spectroscopy of polyenes 156 157
Fluorescent prostaglandin derivatives, tritium-labelled 818
Fogarasi, G. 4 5 8—10(31) 21 152(19) 162(92) 170 171
Foldesova, M. 352(144—146) 357
Foldiak G. 340(84) 341(79) 356
Fonken, G.J. 508(4) 544
Font, J. 17(105 106 112 114) 18(106) 19(112 114) 20(105 106 112 114) 22 23
Foos, J.S. 42(95) 63
Foote, C.S. 919(182) 926
Forbes, J.E. 904(85) 924
Forbes, W.F. 155(30) 170
Force fields 6
Ford, W.T. 745—747(45) 751
Foresman, J.B. 11(61) 22 32(25) 62
Forner, W. 742(43) 751
Forshult, S. 326(6) 354
Forster, P. 237 250(284) 261
Forster, T. 209(181) 258
Fortunak, J.M.D. 372 373(48a 48b) 469
Foss, J.S. 180 183 184 225 228(63) 255
Foster, A. 825(127) 864
Foster, D.F. 498(36) 505 907(104) 924
Fotsch, CA. 536(108) 546
Fowler, R.B. 720(62) 732
Fox, C.M.J. 388 391(96f) 470
Fox, D.J. 32(25) 62
Fox, MA. 232(224) 259 772(24) 774
Fox, N.A. 678(65) 681
Fraenkel, G. 747(56 57) 751
Fraile, J.M. 911(133) 925
Franck — Condon envelopes 176 225
Franck, R.W. 17 19 20(117) 23
Francotte, E. 405(146) 472
Frank, H.A. 243(298) 261
Franke, LA. 845(181) 865
Franken, T. 339(70) 355
Franklin, J.L. 549(19) 611
Fratev, F. 51(138) 64
Freed, K.F. 11 12(60) 13(71) 22
Freeman, G.R. 335(25) 339(73) 341(86 87) 354—356
Freidinger, R.M. 375(55) 469
Fremery, M.I. 920(200) 926
Frenking, G. 17 19 20(118) 23 741(38 39) 743(39) 750
Freyer, A.J. 313(110a) 324
Fridh, C 179(26 46) 180(26) 183(26 126) 184 223 225(26) 255 257
Friedli, H. 892(11) 922
Friedman, L. 522(63) 545
Friege, H. 718(59) 732 967(103) 976
Friere, R. 87(57) 108
Friesen, R.W. 440 444(246d 246e) 477
Frimer, A.A. 915(154) 925
Fripiat, J.G. 15(84) 22
Frisch, M.J. 32(25) 62
Fritz, H. 252(309) 261
Fritzsche, J. 308(97a) 323
Froborg, J. 405(141a 141b) 472
Frohlich, L. 915(160 161) 925
Frolich, J.C. 782(20) 861
Fronczek, F.R. 53(151) 65
Fronda, A. 57(177) 65 949(60 61) 959(60 61 81) 975 976
Frost, D.C. 179(29) 184(136) 185(159) 225(136) 255 257 258
Frostin-Rio, M. 639(78) 651
Fry, A.J. 768(15) 774
Frydman, B. 807(78) 863
Frydrych-Houge, C.S.V. 430(193b) 475
Fu, E.W. 184(149) 236(237 252) 237(281) 249(252) 257 260 261
Fu, G.C. 542(122 123a) 546
Fuchs, P.L. 393(103b) 394(108a 108b) 423(173a) 471 474 906(97) 924
Fueno, T. 722(66) 732
Fugami, K. 633(64) 651
Fuhiwara, K. 445(251) 478
Fujimoto, Y. 272(20) 320
Fujio, M. 872(21a 22) 886
Fujisaka, Y. 236 237(258) 260
Fujisawa, J. 338(57) 355
Fujita, S. 428 429(190e) 475
Fujita, Y. 388(100) 471
Fujiwara, T. 388 392(97b) 471
Fukazawa, Y. 445(251) 478
Fukui, K. 630(47) 651
Fukumoto, K. 276(28d) 320 404(137a) 472 517(42) 544
Fukunaga, T. 42(94) 63 940 942(23 24) 975
Fukuyama, T. 5(38b) 21 48(122) 51(132) 64 158(47) 170
Fullerenes 930 963 “Methanofullerenes”)
Fullerenes, Diels — Alder reaction of 575 581
Fullerenes, structure of 58
Fulscher, M.P. 247 248(301) 261
Fulvenes (see also “Pentafulvenes” “Triafulvenes”)
Fulvenes, structure of 52 53
Fulvenes, thermochemistry of 92 94—98
Fung, B.M. 904(82) 924
Fung, VA. 531(94) 545
Funhoff, D.J.H. 601(172) 616
Funk, R.L. 366 367(28c) 399(124c) 468 472 517(43) 544
Funke, C. 297(74) 322
Furanoid terpenes, synthesis of 669
Furans, Diels — Alder reaction of 575 580 582—586 591
Furans, Paterno — Buchi cycloaddition of 298—305
Furber, M. 459(297 298) 479
Furika, H. 561(63c) 613
Furr, H.C. 783(29) 862
Furukawa, H. 423(177) 474
Furukawa, Y. 5 6(39) 21 150(7) 158 159(60) 161(60 78) 162(60 84 93) 163(93) 164(84 93) 165(93) 166(7 60 84 93 107 119) 167(122) 168(60 93 107) 169(7) 169 171 172
Furuta, H. 561(63c) 613
Furuta, S. 892(8) 922
Fussganger, V. 818(105) 863
Fututa, T. 799(52—55) 862
Fuzioka, A. 452(271) 478
G values 326
Gacs-Baitz, E. 566(71) 613
Gad, A. 964(96) 976
Gadwood, R.C. 415 421(171c) 474
Gaes-Beitz, E. 593(143) 615
Gage, J.R. 440 443(245b) 477
Gahagan, M. 498(36) 505 907(104) 924
Gailliard, T. 918(176) 926
Gailyunas, LA. 907(105) 924
Gajewski, JJ. 851(202—204) 855(203 225 226) 857(225 226 229 230) 866
Galasso, V. 11(53) 21 965 970(99) 976
Galbraith, R. 847(192) 848(193) 866
Galeeva, R.I. 921(205) 926
Gallagher, T. 399(124g) 472 678(65) 681
Gallego, M.G. 278(37) 321
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -dicarbonyls
-dicarbonyls  - and
- and  -labelled, synthesis of
-labelled, synthesis of 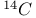 - labelled, synthesis of
- labelled, synthesis of