|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Rappoport Z. (ed.) — The Chemistry of Dines and Polyenes (vol. 1) |
|
|
 |
| Предметный указатель |
Dehydrotosylation, as synthetic procedure for conjugated dienes 368 369
Deiters, U. 559 561 563(52) 613
Dejroongraung, K. 71(llb) 72(llb 14) 77 88(11b) 104 105
deKock, R.J. 268(16a) 320
Del Bene, J. 242(292) 261
Delhalle, J. 2 15(2) 20
Dellepiane, G. 166(115) 172
Delton, M.H. 402(135) 472
DeLucca, G. 45(110) 64 181 185(98) 256
DeLucchi, O. 277(32c) 321
Delugeard, Y. 53(154) 65
Dembek, A.A. 155(37) 170
Demuth, M. 281(41b) 321
Denis, J.-M. 881(36) 886 945(40) 975
Denissen, J.F. 848(194) 866
Denmark, S.E. 455(282a) 479 509(9a 9b) 544
Density fractional theory 3 18
Deoxygenation, reductive, as synthetic procedure for conjugated dienes/polyenes 368—372
Dep, B. 463(303a) 480
Depezay, J.C. 412 414(162c) 473 782(23) 861
DePuy, C.H. 735(8 10) 736(13) 738 739(8) 750
Derguini, F. 141(50b) 147
Derone, A.E. 606(185b) 617
Desai, S.R. 398(118) 471
Descoins, C 454 455(278a) 479
DeShong, P. 417(164c) 473
Desmaele, D. 301(85a 86) 323
Desmond, R. 533(101) 546
Desrosiers, M. 337(45) 355
Dessau, R.M. 631 632(53) 645(53 87) 651 652
Desulphonylation, reductive, as synthetic procedure for conjugated dienes 375 376
Dettmer, G. 535(106) 546
Deuter, J. 44(102—104) 64
Dev, S. 276(30a) 320 453(276b) 479
Devaux, P. 500(43) 505
DeVita, RJ. 666(42) 680
DeVoe, H. 133(41a) 147
Devyatykh, G.G. 802(67) 862
Dewar benzene, structure of 43
Dewar pyrones 268 270
Dewar, M.J.S. 2 17(5) 20 32(23) 62 102(99b) 110 207(178) 225(212) 258 259 630(48) 651
DeWitt, E.J. 717(58) 732
Dfflet, V. 17 19 20(129) 23
Dhanoa, D.S. 627 631 633—635(39) 650
Di- -methane rearrangements 277—280 (see also “Aza-di- -methane rearrangements 277—280 (see also “Aza-di- -methane rearrangements”) -methane rearrangements”)
Diacyloxylation 662 663
Diacyloxylation aerobic 667 668
Dickerson, J.E. 434(209d) 435(218) 476
DiCorato, A. 117 135(12) 146
Dicyanomethyl radical, addition to dienes 621 622
Diechsel-Grau, E. 621 622(11) 650
Dieck, H.A. 438(228) 477
Diederich, F. 56(167) 65 940 945(26a) 971 973(107 108) 975 977
Diederichs, F. 58(180) 65
Diedrich, M.K. 599(163) 602(174) 603(190) 606(186) 607(187) 608(188) 616 617
Dieleman, J. 228 232(215) 259
Diels — Alder reactions (see also “Retro — Diels — Alder reactions”)
Diels — Alder reactions, catalysis of 19 20
Diels — Alder reactions, diastereofacial selectivity in 19
Diels — Alder reactions, effect of pressure on 552—596 603—607
Diels — Alder reactions, effect of pressure on mechanistic aspects of 552—563
Diels — Alder reactions, effect of pressure on synthetic applications of 563—591
Diels — Alder reactions, intramolecular 511—518 603—607
Diels — Alder reactions, mechanism of 17—19
Diels — Alder reactions, mechanism of, isotope effect studies of 776 848—854
Diels — Alder reactions, pincer/domino 573 575
Diels — Alder reactions, regioselectivity in 19
Diels — Alder reactions, site-selectivity in 19
Diels — Alder reactions, solvent effects on 19 20
Diels — Alder reactions, stereoselectivity in 19
Diene chirality rule 117—120 131
Diene rule 139
Dienes (see also ”Bis-dienes” “Butadienes” “Decadienes” “1 “1 “Heptadienes” “Hexadienes” “Norbornadienes” “Norcaradienes” “Octa-dienes” “Pentadienes” “Propelladienes” “Spirodienes”
Dienes cisoid 112 113 120—122 126
Dienes distorted 114 117 118 126—132
Dienes transoid 112 113 131
Dienes, electronic spectra of 236
Dienes, PE spectra of 178—182
Dienes, polymerization of, radiation-induced 343—346
Dienes, radiolysis of in aqueous solntion 327—334
Dienes, radiolysis of in non-aqueous solvents 334—339
Dienes, reactions of, with hydrated electrons 328
Dienes, reactions of, with hydrogen atoms 328
Dienes, reactions of, with hydroxyl radicals 328
Dienes, s-cis 6 7 112—114 126 128—134 141 142
Dienes, s-trans 41 112—114 117 135—137 141 142
Dienes, skewed 131 132
Dienes, synthesis of, 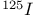 -labelled 844—846 -labelled 844—846
Dienes, synthesis of, 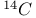 -labelled 827 828 831 832 841—843 -labelled 827 828 831 832 841—843
Dienes, synthesis of, deuterium-labelled 776—802
Dienes, synthesis of, tritium-labelled 818—822
Dienoates see “Eicosadienoates”
Dienones see “Cross-conjugated dienones” “Cycloalkadienones”
Dienophiles, unsymmetrical, in Diels — Alder reaction 18 19
Dienth, W. 34(41) 62
Dienyl radical cations 338
Diercksen, G.H.F. 211(189) 258
Diessel, J. 181; 184 185(92) 256 365(23c 23d) 468
Dietrich, H. 56 57 61(166) 65
Dietz, F. 974(110) 977
Dieue chromophore, 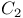 120 120
Dieue chromophore, distortion of 114 117 118
Dieue chromophore, electronic absorption spectrum of 112—114
Dihydrocostunolide, synthesis of 272
Dihydronaphthalenes, structure of 45
Dihydronaphthalenes, thermochemistry of 90 99
Dihydronaphthalenes, [4+4]photocycloaddition of 306 307
Dihydronovanin, synthesis of 272 273
Dihydropyrans, synthesis of 268 270
Diing, L.G. 818(102) 863
Diisko, R.L. 26(1) 61
Dill, J.D. 184(144) 257
Dilworth, B.M. 374(52f) 469
Dimichele, L. 440 441(244c) 477
Dimitrova, BA. 485(9) 504
Ding, R.S. 338(59 60) 355
Ding, S.F. 822(116) 864
Dinne, E. 510(13) 544
Diols, formation of 891
Diols, unsaturated, reductive deoxygenation of 368—372
Dioxythiepines, photocycloaddition of 315 318
Diphenylallyl anions 747 748
Diphenylphosphonyl radicals, addition to dienes 623
Dirac, P.A.M. 152(21) 170
Dirkzwager, H. 770(22) 774
Dispirodecadiene, structure of 42
Ditchfield, R. 78(27) 106
Dittami, J.P. 280(39) 321
Divinyl ketones, cyclization of 508 509
Dixon, D.A. 15(86) 22
Djerassi, C 485 489 490 496(7) 504
Djuric, S.W. 440 441(244a) 477
Do, U.H. 860(238) 867
Dobler, W. 185(165) 258
Docosahexaenoates, deuterium-labelled, synthesis of 780 781
Docosahexaenoic acid,  -labelled, synthesis of 824 825 -labelled, synthesis of 824 825
Dodecahedranes 50
Dodelet, J.P. 335(25) 339(73) 354 355
Doecke, C.W. 181(94) 256
Doering, J.P. 11(64 65) 22
Doering, W.V.E. 39(79) 54(158) 63 65 76 77(23) 78(26) 98(23) 106 179(37) 183 184(117) 211(37) 255 257 559(51) 596(158) 608(189) 613 616 617 627(25 27) 650
Dogan, B. 80(32) 98(82) 106 109 570 573(86) 614
Dogan, B.M.J. 559(51) 560(53) 562(59) 570 573(87) 574(91) 595 596(152) (153) 613 614 616
Dolbier, W.R. 594 595(149) 616
Dolbier, W.RJr. 76 77(23) 78(26) 98(23) 102(100) 106 110 858(231 232) 859(232) 866
Dole, M. 347(109) 351(139 140) 356 357
Dolle, R.E. 410(155c) 415 422(171e) 473 474
Dolman, D. 277(33) 321
Domaille, P.J. 52 53(144) 65
Domalski, E.S. 70(9) 104
| Domcke.W. 211(189) 258
Domelsmith, L.N. 181(84) 225(213) 256 259
Domingues, E. 438 439(234a) 477
Domnin, I.N. 179(41) 255
Domow, R. 621 622(11) 650
Doney, J.J. 424 425(179c 179d) 475
Doning, D. 440 442(244e) 477
Donkersloot, M.C.A. 119(19a 19c) 146 147
Doolittle, R.E. 455(282b) 479
Dore, L. 53 54(159) 65
Dorfman, L.M. 327(9) 354
Dorko, E.A. 56(164 165a 165c) 57 58 61(164) 65 931(2) 974
Dosio, F. 823(122) 864
Dowbenko, R. 522(62) 545
Doxsee, K.M. 427(185) 475
Doyle, A.M. 717(56) 732
Dragonette, K.S. 33(34) 62
Drake, A.F. 116 117 132 133(10) 146
Drake, S.R. 791(42) 862
Dreiding, A. 43(97) 63
Drew, M.B.G. 586(114) 615
Dromzee, Y. 376(61) 469
Drouin, J. 181 184(97) 256
Drummond, G.S. 847(192) 866
Dube, D. 388 390(96d) 470
Dubois, J.-E. 631 632 643 649(54b) 651
Dubuis, R. 453(276a) 479
Dudis, D. 15(85) 22
Dudis, D.S. 15(80) 22
Dudley, G.K. 344(94) 356
Duhamel, L. 382(83f-h 84—86) 384(83f-h) 418(86) 419(83f) 470
Duhamel, P. 382(83f 83g 86) 384(83f 83g) 418(86) 419(83f) 470
Duke, AJ. 401(132b) 472
Dumer, G. 266(10a 10b) 319
Dunbar, R. 229(218—220) 230(219 220) 236(237 238) 249(238) 259 260
Dunbar, R.C. 179(25) 184(149) 236(244 245 252 267 272) 237(281) 248(267) 249(245 252) 255 257 260 261
Duncan, J.H. 377 378(69a) 469
Dunitz, J.D. 37(57) 58 60(181) 63 65 964(97) 976
Dunkin, I.R. 236(263) 237(263 285) 248(285) 249(263 285) 260 261
Dunogues, J. 673(57c) 681
Dunston, J.M. 289(55b) 322
Duplantier, AJ. 420(166c) 474
Dupont, G. 141(52a) 147
Dupous, M. 4(12 17) 21
Dupuis, M. 15(79) 22 31(15—17) 35(17) 61 166(109) 172
Duraisamy, M. 136(44a 44b) 147
Duran, M. 16(93) 17 19 20(123) 22 23
Durig, J.R. 6(41) 21
Durst, T. 404(137c) 472 610(201) 617
Duthaler, R. 298(77b) 322
Dykstra, C.E. 16(92) 22
Dynamic coupling 123 133
Dyomin, P.M. 813(98) 863
Dzhemilev, U.M. 907(102) 924
D’Aloisio, R. 912(138) 925
d’Angelo, J. 17 19 20(108) 22
E- , synthesis of 844 845 , synthesis of 844 845
Easwaran, K.R.K. 141(51a) 147
Eaton, D.F. 179(30) 255
Eberbach, W. 181(87) 256
Eberhardt, A. 34(42) 62 440 443(245c) 477
Ebrey, T.G. 141(50a) 147
Ebsworth, E.A.V. 179(43) 255
Echegoyen, L. 772(24) 774
Echinocandin B,  - and - and  -labelled, synthesis of 801 802 -labelled, synthesis of 801 802
Echter, T. 958(79) 976
Eckell, A. 274(26) 320
Eckert, CA. 554(34) 556(39) 557(40 41) 558(47a 47b) 612
Eckert-Maksic, M. 26(5) 61 177(12) 180(12 65) 181(65) 221(208) 254 255 259
Eckrich, T.M. 439(239 240a) 477
Edenborough, M.S. 606(185b) 617
Edge effects, in long polyenes 10
Edmiston, C 220(205) 259
Edmunds, J.E. 440(248) 478
Edwards, J.M. 126 134(36) 147
Edwards, M.P. 388 389(96a) 470
Edwards, P.D. 894(22) 922
Efimov, I.B. 352(142) 357
Eftenberger, F. 411(155f) 473
Egawa, Y. 232(228) 259
EgHnton, G. 493(18) 504
Egmond, M.R. 917(172) 925
Ehrenson, S. 688(16) 731
Eichel, W. 621 622(11) 650
Eicher, T. 379(76a) 470
Eickhoft, D.J. 907(113) 924
Eicosadienoates, deuterium-labelled, synthesis of 778
Eicosatetraenoic acids,  -labelled, synthesis of 825 826 -labelled, synthesis of 825 826
Eicosatrienoates, deuterium-labelled, synthesis of 778—780
Eisenstein, O. 17 19 20(116) 23
Eiter, K. 364(24b) 468
Ejiri, E. 428 429(190e 190f) 475
El Tayar, N. 802(64) 862
El-Dim, G.N. 609(193) 617
El-Din, G.N. 588(122) 610(200) 615 617
El-Sayed, MA. 150 151(10) 170 183(119) 257
Eland, J.H.D. 175(9) 179 184(18) 254
Elango, V. 417(164c) 473
Eldik, R.van 548(4 6) 611
Electrical effects 687—702
Electrical effects, classification of 690 692—698
Electrical effects, parameters for 698—702 728 729
Electrical properties, molecular 15 16
Electrocyclic reactions 507—510
Electrocyclic reactions photochemical 265—276
Electrocyclic reactions, effect of pressure on 597 599 602
Electron affinity 733 734
Electron capture detection 500
Electron correlation 3
Electron energy loss spectroscopy, in structure determination of dienes 486 488
Electron nuclear double resonance spectroscopy 499
Electron spin resonance spectroscopy of oligomer radicals 344
Electron spin resonance spectroscopy of polymers 349
Electron spin resonance spectroscopy of radiolytic radical cations 337 338
Electron-electron interactions 214
Electronic spectroscopy 228—239
Electronic spectroscopy of dienes 236
Electronic spectroscopy of polyenes 236—238
Electronic transitions 11
Electrophilic aromatic substitution, intramolecular 291 292
Elix, JA. 366(27) 468
Eller, B.C. 845(182) 865
Ellerman, T. 339(67) 355
Ellis, D.E. 871(15) 886
Ellison, G.B. 11(61) 22 735 738 739(8) 750
Ellsworth, R.L. 840(172) 843(176) 865
Elsasser, D. 182(110 113) 257
Elvidge, JA. 822(115) 864
El’yanov, B.S. 552(30) 592(141) 598(162) 612 615 616
Ema, K. 350(133 134) 357
Emken, E.A. 778(6 9) 861
Emram, J. 855 857(225) 866
Enantiodivergent synthesis 663
Enchev, V. 51(138) 64
Enders, D. 387(94) 470
Endo, Y. 51(130) 64
Endoperoxides, formation of 891
Ene reactions, intramolecular 518—521
Ene reactions, metallo 520
Ene-allenes, cyclization of 541 542
Eng, W. 809 810(87a 87b) 863
Engberts, J.B.F.N. 591(133) 615
Engelhardt, L.M. 182(109) 257
England, W. 220(205) 259
Englert, G. 493(19) 504(54) 505
English, J.H. 236(269) 260
Engman, L. 366 367(28d) 468
Enkelman, V. 34(42) 62
Enkelmann, V. 440 443(245c) 477
Enoki, T. 963(89) 976
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -methane rearrangements
-methane rearrangements 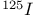 -labelled
-labelled 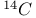 -labelled
-labelled 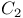
 -labelled, synthesis of
-labelled, synthesis of  , synthesis of
, synthesis of  - and
- and  -labelled, synthesis of
-labelled, synthesis of