|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Rappoport Z. (ed.) — The Chemistry of Dines and Polyenes (vol. 1) |
|
|
 |
| Предметный указатель |
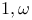 -Bis(diarylethenyl)alkanes, radiolysis of 338 -Bis(diarylethenyl)alkanes, radiolysis of 338
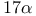 -(2-Iodoett)enyl)androsta-4,6-dien- -(2-Iodoett)enyl)androsta-4,6-dien-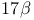 -ol-3-one, -ol-3-one, 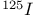 -labelled, synthesis of 845 846 -labelled, synthesis of 845 846
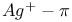 -complexation, in separation of dienes/polyenes 485 -complexation, in separation of dienes/polyenes 485
 58 930 963 58 930 963
![$[Cys-\sidset{^{14}}{}CO]LTC_4$](/math_tex/89e1ca9467ce0124a3f41d7092ea88f382.gif) , synthesis of 830 831 , synthesis of 830 831
 -Carbonyl radicals 644 -Carbonyl radicals 644
 -Farnesene, deuterium-labelled, synthesis of 791—793 -Farnesene, deuterium-labelled, synthesis of 791—793
 -Lumieolehieine, synthesis of 268 269 -Lumieolehieine, synthesis of 268 269
 -Phellandrene, photocyeloaddition of 274 275 -Phellandrene, photocyeloaddition of 274 275
 -Terpinene, ring cleavage of 892 -Terpinene, ring cleavage of 892
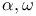 Dialkylpolyenes, absorption spectra of 155 Dialkylpolyenes, absorption spectra of 155
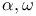 Dialkylpolyenes, Raman spectra of 166—168 Dialkylpolyenes, Raman spectra of 166—168
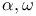 -Diphenylpolyenes, absorption spectra of 155 156 -Diphenylpolyenes, absorption spectra of 155 156
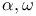 -Dithienylpolyenes, absorption spectra of 155 156 -Dithienylpolyenes, absorption spectra of 155 156
 -Angelica lactone, Diels — Alder reaction of 19 -Angelica lactone, Diels — Alder reaction of 19
 -Carotene, deuterium-labelled, synthesis of 784 786 -Carotene, deuterium-labelled, synthesis of 784 786
 -Carotene, structure of 150 -Carotene, structure of 150
 -Carotene, thermochemistry of 87 -Carotene, thermochemistry of 87
 -Vetivone, synthesis of 282 283 -Vetivone, synthesis of 282 283
 -Laetones, fonnation of 644 645 -Laetones, fonnation of 644 645
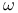 -Iodoundecenyl cholesteryl ether, -Iodoundecenyl cholesteryl ether, 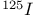 -labelled, synthesis of 846 -labelled, synthesis of 846
 -Allyl complexes 654 661 662 676 -Allyl complexes 654 661 662 676
 -Olefln complexes 654 -Olefln complexes 654
 -Orbitals, manifold of 214 -Orbitals, manifold of 214
 -Orbitals, noneonjugated, interaction between 215—228 250—254 -Orbitals, noneonjugated, interaction between 215—228 250—254
 -Orbitals, two-centre 201 202 -Orbitals, two-centre 201 202
(Diene)palladium(II) complexes 654
1,1-Divinylcyclopropane, structure of 26—28
1,1-Divinylethylene, thermochemistry of 93
1,2-Dibromides, dehydrobromination of 364 365
1,2-Divinylcyclohexanes, oxidation of 658—660
1,3,5,7,9-Decapentaene, structure of 31
1,3-Butadiene, acidity of 735 736
1,3-Butadiene, conformation of 6 7 113 114 141 158—161
1,3-Butadiene, copolymerization with styrene 346
1,3-Butadiene, Diels — Alder reaction of 17—19
1,3-Butadiene, electronic transitions in 141 142
1,3-Butadiene, excited states of 11—13
1,3-Butadiene, force constants for 6
1,3-Butadiene, geometry of 4 5 114
1,3-Butadiene, harmonic vibrational frequencies for 5
1,3-Butadiene, MOs of 141 142
1,3-Butadiene, photodimerization of 296
1,3-Butadiene, polymerization of 343 345 346
1,3-Butadiene, radiolysis of 335
1,3-Butadiene, structure of 31 32 35
1,3-Butadiene, structure of, compared to radialenes 55 56
1,3-Butadiene, symmetry in 141—143
1,3-Butadiene, vibrational spectra of 158—161
1,3-Cyclohexadiene, Diels — Alder reaction of 566 568
1,3-Cyclohexadiene, polymerization of, radiation-induced 343 344
1,3-Cyclooctadienes, cyclization of 508
1,3-Cyclooctadienes, radiolysis of 335
1,3-Dienes (see also “1 “1
1,3-Dienes, 1,4-chloroacetoxylation of 663 664
1,3-Dienes, 1,4-diacetoxylation of 662 663
1,3-Pentadiene, acidity of 740
1,3-Pentadiene, radiolysis of 335
1,4-Benzoquinones, as oxidizing agents 658 662 666 670 675 676
1,4-Benzoquinones, Diels — Alder reaction of 564 565
1,4-Chlorolactonization 668
1,4-Cyclohexadiene, reactions with hydrogen atoms 328
1,4-Dialkoxylation 666
1,4-Dienes (see also “1
1,4-Dienes, palladium-catalysed oxidation of 660 661
1,4-Dienes, photorearrangement of 277—280
1,4-Oxyamination, palladium-catalysed 670
1,4-Oxychlorination 669 675
1,4-Oxylaetonization 668
1,4-Pentadiene, structure of 26 27
1,4-Pentadiene, thermochemistry of 71
1,5-Cyclooctadienes, palladium-catalysed oxidation of 657
1,6-Diphenylhexatriene, thermochemistry of 89
1-Phenylethyl radicals, addition to dienes 624
10,11-Dibromodibenzosuberone, tritium-labelled, synthesis of 821 822
13-cis-Retinoic acids (see also “4-Oxo-13-cis-Retinoic acid”)
13-cis-Retinoic acids synthesis of, 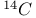 -labelled 834 836 -labelled 834 836
13-cis-Retinoic acids synthesis of, deuterium-labelled 783 784
15-Deoxy-16-hydroxy-16-methyl-5-thiaprosta-glandin 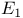 methyl esters, deuterium-labelled, synthesis of 797—799 methyl esters, deuterium-labelled, synthesis of 797—799
17-Amino-22-(4’-azido-3’-iodophenacyl)-17-demethoxygeldanamycin, 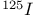 -labelled, synthesis of 847 -labelled, synthesis of 847
2,3-Dichlorobutadiene, polymerization of, radiation-induced 343
2,3-Dimethylbutadiene, polymerization of, radiation-induced 343 344
2,4-Dimethylpentadiene, conformation of 7
2,5-Cyclohexadienones, photorearrangement of 280—285
2,6-Cycloheptadienones, photorearrangement of 286 287
2,7-Cyclooctadienones, photorearrangement of 285 286
2-Hydroxyalkyl-4-pyrones, photorearrangement of 290 291
3,4-Dimethylenecyclobutene, thermochemistry of 93 99 100
3-Hydroxy-4-pyrones, photorearrangement of 288 289
3-Hydroxyalkylpyrroles, synthesis of 305 306
4-Hydroxycyclohexene, radiolytic formation of 331
4-Hydroxypyrylium ions, photorearrangement of 289
4-Oxo-13-cis-retinoic acid, 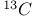 -labelled, synthesis of 805 -labelled, synthesis of 805
9-cis-Retinoic acids, tritium-labelled, synthesis of 808 809
Ab initio calculations 3
Ab initio calculations for Diels — Alder reaction 17—19
Ab initio calculations for evaluation of contributions to CD 124
Ab initio calculations for excited states 13 14
Abbott, P.A. 871(17) 886
Abdi-Oskoui, H. 557(42) 612
Abe, S. 827(146) 864
Abecassis, J. 398(121 122) 471
Abell, P.I. 627 634 636(35) 650
Abelman, M.M. 433(211) 476
Abeln, J. 605(183) 616
Abels, J. 590(129d) 615
Aben, R.W. 402 403(134c) 472
Aben, R.W.M. 586(115) 592(140) 615
Abiko, A. 420(166b) 474
Abram, T.S. 874(27) 886
Abrams, G.D. 531(94) 545
Absorption spectroscopy 151
Absorption spectroscopy of diene chromophore 112—114
Absorption spectroscopy of polyenes 155—158
Absorption spectroscopy of radiolytic intermediates 328—333
Acetylene, acidity of 735
Acetylene, deprotonation energy of 737 738
Achenbach, H. 494(22) 505
Achi, S.S. 639(78) 651
Achiba, Y. 179(15) 254
Achini, R.S. 532(95) 545
Acidity, gas-phase 733 734
Acidity, gas-phase of allyl hydrogens 739 740
Acidity, gas-phase of vinyl hydrogens 735—737
Acquardo, M. 298(78) 322
Acrolein, Diels — Alder reaction of 20
Acrylonitrile, radical addition to 625 626 648
Activation volume 548—552
Activation volume relationship with ring size 603 608 609
Acuna, A.U. 423(172a) 474
Adachi, N. 660(27) 680
Adam, M. 34(42) 62 440 443(245c) 477
Adam, W. 277(32c) 321 905(89) 911(128—131) 914(151) 924 925
Adamczak O. 36(50) 62 70(8 9) 104
Adams, J. 410(155a) 473 496(27) 505
Adger, BJ. 458(295) 479
Adjacency matrix 201 204
Adlington, R.M. 377(68c) 469 849(197) 866
Adlof, R.O. 778(6 9) 861
Aebischer, J.N. 248(304) 261
Afonso, C.A.M. 430(193a) 475
Afzal, J. 401(130) 472
Agarrabeita, A.R. 278(37) 321
Agarwal, D.D. 907(103) 924
Ager, D.J. 424(178e 178i) 475
Aglietto, M. 158(55) 171
Agmon, I. 947(48) 975
Agnel, G. 436(223a) 477
Ahamed, Z. 446 451(257f) 478
Ahem, D. 860(238) 867
| Ahlrichs, R. 40(85) 63
Ahmad, S. 378(73) 469 898(50) 923
Ahmed, G. 315 317(120) 324
Ahuja, V.K. 781(13) 861
Aihara, J. 51(137b) 64
Aihara, M. 788(35) 862
Aistars, A. 315(112b) 324
Aizenshtat, Z. 492 493 503(15) 504 920(191) 926
Akagi, J. 913(141) 925
Akai, S. 408(154c) 473
Akasaka, T. 916(164) 925
AkasaM, Y. 313(109d 109f 109h) 324
Akermark, A. 423(175) 474
Akermark, B. 416(164b) 423(174) 434(209c) 473 474 476 653(lb) 661(30) 679 680
Akhunova, V.R. 921(205) 926
Al-Jobomy, M.I. 175(6) 254
Al-Laham, M.A. 32(25) 62
Alami, M. 438 439(233 234a) 455(283a) 477 479
Albert, B. 182(110) 257
Albert, I.D.L. 15(89) 22
Alberts, A.W. 827(144) 864
Alberts, I.L. 35 36(46a) 62 161(76) 171
ALBERTS, IA. 4(15) 21
Albrecht, A.C. 153(25) 170 243(298) 261
Albright, T.A. 174(1) 254
Alcock, H.R. 343(92) 344(94) 356
Alder, K. 518(44) 544
Aldol products 299
AlexaMs, A. 449(267a) 456(288) 478 479
Alfassi, Z.B. 332(18) 354
Algrim, DJ. 745 746(48) 751
Alkylidenecycloalkanes (see also “Methylenecycloalkanes”)
Alkylidenecycloalkanes, structure of 50—52
Alkylidenecycloalkenes (see also “Methylenecycloalkenes”)
Alkylidenecycloalkenes structure of 51—54
Alkynes (see also “Acetylene” “Polyalkynes”)
Alkynes isomerization of 456 457
Alkynyl cations 869
all-trans-Menaquinone-4, 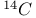 -labelled, synthesis of 837 -labelled, synthesis of 837
Allan, M. 184(145) 236(268) 257 260
Allauddin, M.M. 399(124h 124i) 401(124i) 472
Allen, C.F.H. 401(132a) 472
Allen, F.H. 41(90) 63
Allen, W.S. 770(20) 774
Allenby, G. 808(83) 863
Allenes (see also “Chlorotriarylallenes” “Cumulated
Allenes epoxidation of 905 906
Allenes formation of 265
Allenes oxidation of, palladium-catalysed 677—679
Allenes oxidation of, with ozone 921
Allenes oxidation of, with singlet oxygen 915 916
Allenes radiolysis of 338
Allenes reduction of, selective 466 467
Allenes thermochemistry of 72
Allenic polyenes see “Cumulated polyenes”
Allenyl cations 869 870
Allenyl cations cycloadditions of 877—881
Allenyl cations ferrocenyl-substituted 874 875
Allenyl cations generation of 870—881
Allenyl cations identification of 881 882
Allenyl halides, photolysis of 870 871
Allinger, N. 749(67) 751
Allinger, N.L. 38(67) 39(77) 63 70 71 88(7) 104 563(64a) 613 686(4) 730
Allred, A. (29) 731
Allyl anions (see also “Diphenylallyl anions” “Triphenylallyl
Allyl anions in solution 744—750
Allyl anions stabilization of 740—743
Allyl anions structure of 741 742
Allyl anions theoretical studies of 740—744
Allyl carbanions 673
Allyl cations, trapping of 647 648
Allyl radical cyclization 627—630
Allyl radicals 329 335 339 350
Allyl radicals dimerization of 640—643
Allyl radicals, electron affinity of 739
Allyl radicals, oxidation of 646—649
Allyl radicals, stabilization energy for 627
Allyl radicals, trapping of, with closed-shell molecules 634—637
Allyl radicals, trapping of, with radicals 637—640
Allylic acetates, decarboxylative eliminations of 372—374
Allylic alcohols, formation of 891
Allylic axial chirality rule 120—126
Allylic axial chirality rule diene/olefin picture of 125—127 131 132
Allylic dihalides, dehalogenation of 366
Allylic halides, dehydrohalogenation of 364 366
Allylic hydrogens, abstraction of 328
Allylic hydrogens, gas-phase acidity of 739 740
Allylic nitroacetates, reductive elimination reactions of 377
Allylic substituents, contribution to CD intensity 123—126
Allylic sulphones, as precursors of conjugated dienes/polyenes 394 396
Allyllithiums 744 745 747
Allylpotassiums 746 747
Allylsilanes, cationic cyclization of 533—536
Allylsilanes, reactions of 673 674
Almenningen, A. 40(84) 42(92) 53 54(152) 63 65 158(45) 170
Alonso, D. 17 19 20(112 114) 23
Alonso, I. 393(104) 471
Alper, H. 456(286 287) 479
Alshuth, T. 150 151(12) 170
Alteramide A, intramolecular [4+4]photocycloaddition of 308
Alvarez, J. 430(192) 475
Alvarez, R. 446 451(257g) 478
Amann, CM. 290(64) 322
Amano, A. 18(132a) 23
Amat Gueriie, F. 423(172a) 474
Amberg, W. 897(44) 923
Ambident cations 871
Ambiphilic radicals 648
Amide ions, reactions of 735 736
Amiich, M.J.Jr. 549(22d) 612
Amim, E.von 347(116) 356
Amit, A. 49(124) 64
AMl method, in study of Diels — Alder reaction 18
Amould, D. 394(105e) 471
Amrein, W. 56(167) 65 940 945(26a) 975
Amrich, M.J. 601(170) 616
Amstrup, B. 31(14) 61
Anastasia, M. 893(15) 922
Anastassiou, A.G. 180 183(76) 256
Ancel, J.-E. 382 384(83h) 470
Andersen, B. 49(123) 64
Andersen, N.H. 526(76a-c) 545
Anderson, D.R. 402 403(134b) 472
Anderson, R. 424(178(1 475
Anderson, RJ. 532(95) 545
Anderson, W.S. 346(100) 356
Andersson, K. 243(296) 261
Andersson, P.G. 668(49a 49b 50) 669(51) 670(52 53) 672 673(54a 54b) 675(58a 58b 59) 676(58b) 677(60) 680 681
Andino, J.M. 922(219) 926
Ando, W. 916(164) 925
Andre, C 388 390(96d) 470
Andre, J.M. 2 15(2) 20
Andreini, B.P. 452(269c) 478
Andres, J.L. 32(25) 62
Andres, J.M. 15(84 87) 16(87 93) 22 371(44) 468
Andrewes, A.G. 501 504(49) 505
Andrews, A.G. 494(23) 505
Andrews, G.C. 812(94) 863
Andrews, L. 233(232) 236(250 253 255 263 264) 237(263 264 276 277 285) 248(250 264 285) 249(250 263 264 285) 250(306) 260 261
Andrews, R.C. 394(105b) 471
Andriamialisoa, Z. 368 369(31b) 380(79 80) 468 470
Andzelm, J. 17 18(98) 22
Anet, E.F.L.J. 791(41) 862
Anet, F.A.L. 81(35a) 107
Anet, R. 405(142) 472
Angell, R. 424(180) 475
Anghoh, A.G. 408(154c) 473
Angus, R.O.Jr. 947(57) 975
Anh, N.T. 17 19 20(116) 23
Annulenes, chemical shifts for 484
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



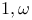 -Bis(diarylethenyl)alkanes, radiolysis of
-Bis(diarylethenyl)alkanes, radiolysis of 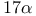 -(2-Iodoett)enyl)androsta-4,6-dien-
-(2-Iodoett)enyl)androsta-4,6-dien-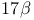 -ol-3-one,
-ol-3-one, 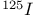 -labelled, synthesis of
-labelled, synthesis of 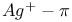 -complexation, in separation of dienes/polyenes
-complexation, in separation of dienes/polyenes 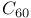
![$[Cys-\sidset{^{14}}{}CO]LTC_4$](/math_tex/89e1ca9467ce0124a3f41d7092ea88f382.gif) , synthesis of
, synthesis of  -Carbonyl radicals
-Carbonyl radicals 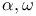 Dialkylpolyenes, absorption spectra of
Dialkylpolyenes, absorption spectra of  -Angelica lactone, Diels — Alder reaction of
-Angelica lactone, Diels — Alder reaction of  -Laetones, fonnation of
-Laetones, fonnation of 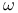 -Iodoundecenyl cholesteryl ether,
-Iodoundecenyl cholesteryl ether,  -Allyl complexes
-Allyl complexes 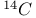 -labelled
-labelled 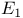 methyl esters, deuterium-labelled, synthesis of
methyl esters, deuterium-labelled, synthesis of 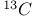 -labelled, synthesis of
-labelled, synthesis of