|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Branden C., Tooze J. Ч Introduction to protein structure |
|
|
 |
| ѕредметный указатель |
Isotypes 300
Jacob, Francois 143
James, Michael 210
Jansonius, Hans 52
Janus protein 369T 370
Jelinski, Lynn 290
Jelly roll barrel in two  sheets 78 sheets 78
Jelly roll barrel, canonical 335 336F
Jelly roll barrel, formation 77Ч78
Jelly roll barrel, viruses 335 335F
Jelly roll barrel, VP1 of rhinovirus 337 338F
Jelly roll domain, SV40 and polyomavirus VP1 342
Jelly roll motif 77
Jelly roll motif, formation/folding 77Ч78 77F
Jelly roll motif, hemagglutinin 80 81F
Jelly roll structure, picornaviruses 335 336F
Jelly roll structure, spherical plant viruses 335Ч337 335F 336F
Johnson, Louise 106
Jones, Alwyn 69
Jun 191 192 199
jun gene 199
Jun, amino acid sequence 192F
Junction diversity, immunoglobulins 302
Jurnak, Frances 255
Kallikrein 362
Kaptein, Robert 164 181
Karle, Jerome 379
Kendrew, John 13 13F 14 370F 379Ч381 380F
Kent, Stephen 9
Keratins 287
Kim, Peter 96
Kim, Sung-Ho 106 255
Kinderstrom-Lang, Kai 28
Kinemage Supplement 23
Kinetic factors, protein folding 91Ч92
Klevit, Rachel 177
Klug, Aaron 176 181 183 327 330
Koshland, Daniel 113
Kossiakoff, Anthony 267
Kraut, Joseph 215
Kretsinger, Robert 24 25
Kringle domains 29F
Kuhlbrandt, Werner 241
Kunitz domains 361 361F
Kunitz domains, inhibitors 361
Kunitz domains, phage-optimized sequences 362T
Kuriyan, John 273 276
Kyte, J. 245
L amino acids 5 9
L subunit, photosynthetic reaction center 235 236Ч237 246F
L subunit, photosynthetic reaction center, conservation between species 246Ч247
L subunit, photosynthetic reaction center, pigments bound to 237Ч239
L-form of amino acids 5 9
Lac operon 143 146
Lac repressor 143Ч145
Lac repressor, binding to major and minor DNA grooves 143Ч145 145F
Lac repressor, helix-turn-helix motif 144 145F
Lac repressor, subunit structure 144 144F
Lac repressor, V-shape tetrameric structure 144 145F
LACI-D1 (Lipoprotein-associated coagulation inhibitor D1) 361 362 362T
Lactate dehydrogenase, Rossman fold 47
Ladner, Robert 362
Lambda bacteriophage see УBacteriophage lambdaФ
Laue diffraction picture 374F 376
Lazarus, Robert 362
Lens, crystallin structure 74 74F
Lesk, Arthur 22F 23 42 311
Leucine zippers 175 191Ч193 202
Leucine zippers in b/HLH/zip family 196F 199Ч200
Leucine zippers, definition 192
Leucine zippers, dimerization interactions 191Ч193
Leucine zippers, globular proteins using coiled coils 287
Leucine zippers, heterodimers 192Ч193 193F
Leucine zippers, side chain interactions 192 193F
Leucine, structure 6F
Leucine, x-ray diffraction data 382
Leucine-rich motifs 47 55 56F
Leucine-rich motifs,  -horseshoe fold 55Ч56 -horseshoe fold 55Ч56
Levinthal, Cyrus 91
Lewis, Mitchell 143
LH1 and LH2 see УLight-harvesting complexesФ
Ligand-binding sites,  helix 16 helix 16
Ligand-binding sites, orientation importance 270 (see also УReceptorsФ)
Light chains see under УImmunoglobulin(s)Ф
Light-harvesting complexes 240Ч241
Light-harvesting complexes, LH1 241
Light-harvesting complexes, LH1, antenna protein ring 242Ч244 243F
Light-harvesting complexes, LH2 241
Light-harvesting complexes, LH2, circular ring of chlorophyll 241 241F 242F 243
Liljas, Lars 339 340
Linker regions, 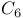 -zinc cluster family binding to DNA 190Ч191 190F -zinc cluster family binding to DNA 190Ч191 190F
Linker regions, GAL4 binding to DNA 189
Linker regions, growth hormone binding to prolactin receptor 270 270F
Linker regions, transducin  256 256
Lipid-binding proteins 70
Lipids, membrane 223 246Ч247 253
Lipscomb, William 60
Loop regions 21Ч22
Loop regions in  barrels 49 barrels 49
Loop regions in 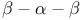 motif 28 motif 28
Loop regions, b/HLH family 200
Loop regions, b/HLH/zip family 200
Loop regions, carboxypeptidase 61 61F
Loop regions, CDK2(T-loop) 108
Loop regions, chymotrypsin 211 211F 212
Loop regions, complementarity determining regions 311
Loop regions, GroES 102
Loop regions, growth hormone receptor 267 267F
Loop regions, hairpin 21Ч22 21F
Loop regions, homeodomains 160
Loop regions, immunoglobulins 304Ч305 304F 305Ч306
Loop regions, model building from x-ray diffraction data 383
Loop regions, movements time scale 105
Loop regions, MyoD 197
Loop regions, neuraminidase 71 71F
Loop regions, omega loop 22
Loop regions, p53 169Ч170 171 171F
Loop regions, prediction 21
Loop regions, Ras protein 255Ч256
Loop regions, serpins 111
Loop regions, spherical viruses 335Ч336
Loop regions, three-dimensional structure 21
Loop regions, variable in homologous proteins 349Ч350
Loop regions, УopenФ and УclosedФ conformations 22
Low, Barbara 27
Lysine, GAM binding to DNA 188 189F
Lysine, recognition helix of glucocorticoid receptor 184Ч185
Lysine, specificity of serine proteinases 213
Lysine, structure 6F
Lysine, trypsin mutation 215
Lysozyme, antilysozyme complex, structure 310Ч311 310F
Lysozyme, Fab binding 309Ч310 310F
Lysozyme, folding pathways 95Ч96 95F
Lysozyme, structure 310Ч311
Lysozyme, T4 bacteriophage see УBacteriophage T4Ф
Lytic-lysogenic cycle switch 130Ч131 130F 133
M subunit, photosynthetic reaction center 235 236Ч237 246F
M subunit, photosynthetic reaction center, conservation between species 246Ч247
M subunit, photosynthetic reaction center, pigments bound to 237Ч239
MacKinnon, Roderick 232 234
Magnesium,  activation 258 activation 258
Magnesium, GTP linking to Ras protein by 255 255F
Magnesium, LH2 light-harvesting complex 241
Main-chain, formation 4 4F
Main-chain, modeling of protein structures 349
Main-chain, polarity 14
Major histocompatibility complex 300 (see also УMHC moleculesФ)
Malaria, resistance and sickle-cell hemoglobin 43Ч45 44F
Maltoporin 230
Mandelate racemase 54Ч55 54F
| Mandelate, conversion to benzoate 54Ч55 54F
Mariuzza, Roy 317
Mat  162 162
Mat 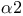 gene, homeodomain 162 162F gene, homeodomain 162 162F
Mat 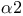 repressor 160 repressor 160
Mat 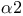 -Mat al complex 163 163F -Mat al complex 163 163F
Matthews, Brian 132 134 354 355
MAX 175 192F
Max, binding to DNA 200F
Max, heterodimer with Myc 199
Max, homodimers 199Ч200
Max, monomer structure 200F
Max, sequence-specific interactions with DNA 201 201F
Mayo, Stephen 367
Mcm1 162
McPherson, Alexander 312
Melting temperature (Tm) 354 356F
Membrane fusogen, hemagglutinin as 80
Membrane lipids 223 246Ч247 253
Membrane proteins 223Ч250
Membrane proteins, crystallization, difficulties 224
Membrane proteins, crystallization, novel methods 224Ч225
Membrane proteins, functions 224
Membrane proteins, signal transduction 251
Membrane proteins, solubilization by detergents 224 225F
Membrane proteins, two-dimensional crystals and EM 225Ч226 226F
Membrane proteins, types 223 223F
Membrane-bound proteins,  helices 35 helices 35
Membranes 223
Membranes, functions 224
Mengo virus 333 336
Menten, Maud 206
Met repressor 175
Metal atoms, in proteins 11 11F
Metallo proteinases 205
Metallo proteins 11
Methallothionein, NMR and x-ray, crystallography comparison 391
Methionine, structure 7F
Methylmalonyl-coenzyme A mutase,  , barrel domain 50Ч51 50T , barrel domain 50Ч51 50T
MHC genes 314Ч315
MHC genes, polymorphism 315
MHC molecules 300 312Ч313
MHC molecules, antigen recognition 314Ч315 316
MHC molecules, Class I 300
MHC molecules, class I, antigen-binding site 314
MHC molecules, class I, peptide binding 315F 316
MHC molecules, class I, peptide complexes 318 318F
MHC molecules, class I, structure 312 313 313F
MHC molecules, Class II 300
MHC molecules, class II, domains 315
MHC molecules, class II, peptide binding 315Ч316 315F
MHC molecules, domains 313Ч314 313F
MHC molecules, peptide complex as ligand for T-cell receptor 318 318F
MHC molecules, structures 312Ч313
MHC molecules, synthesis 316
Michaelis Ч Menten equation 206F
Michaelis Ч Menten scheme 206 206F
Michaelis, Leonor 206
Michel, Hartmut 234 241
microtubules 284
Milligan, Ronald 295
Mineralocorticoid receptor 181F
Model building, antigen-binding sites of immunoglobulins 349Ч350
Model building, Cro-DNA interactions 134Ч135
Model building, hypervariable regions, immunoglobulins 349
Model building, x-ray diffraction data 381Ч382 382F
Modeling of protein structures 349
Molecular chaperones see УChaperonesФ
Molecular disease see УSickle-cell anemiaФ
Molecular dynamics simulations 105
Molten globular proteins 89 92 92F
Molten globular proteins, barnase folding intermediate 94
Monoclonal antibodies, CDR conformation prediction 350 350F
Monod, Jacques 113 117 142 143
Monomeric proteins 29
Motifs 13Ч34 29
Motifs in barrel and sheet structures 47Ч48 49F
Motifs, 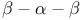 motif see У motif see У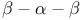 motifФ motifФ
Motifs, combined into domains 29 30
Motifs, Greek key see УGreek key motifФ
Motifs, hairpin  see УHairpin see УHairpin  motifФ motifФ
Motifs, jelly roll see УJelly roll motifsФ
Motifs, simple 24Ч26
Motifs, simple, combination into complex motifs 30Ч31 (see also У helicesФ У УLoop УOther helicesФ У УLoop УOther
Muconate lactonizing enzyme 54 54F
Muirhead, Hilary 51
Multimeric proteins 29
Multiple isomorphous replacement (MIR) 379Ч380
Multiwavelength Anomalous Diffraction (MAD) 381
Muramidase, bacterial 39 39F
Muscle contraction 292
Muscle contraction, ATP role 296Ч297
Muscle fibers 290Ч291
Muscle fibers, thick and thin filaments 290 291 УMyosinФ)
Mutagenesis, oligonucleotide-directed 359F
Mutagenesis, random 359 359F
Mutagenesis, site-directed 163Ч164
Mutations, DNA shuffling method 365Ч366
Mutations, enzyme evolution 55
Mutations, point 366
Mutations, protein folding studies 93Ч95
Myc 191
myc gene 199
Myc, heterodimer with Max 199
Myeloma proteins 309
MyoD 197
MyoD,  helix region 197 198Ч199 helix region 197 198Ч199
MyoD, binding to DNA 198F
MyoD, dimerization region structure 197F
MyoDsequence-specific interactions with DNA 201
Myofibrils 291F
Myogenic proteins 197
Myoglobin as  domain structure 35 domain structure 35
Myoglobin, breathing of molecule 105
Myoglobin, globin fold in 40
Myoglobin, oxygen binding 105
Myoglobin, structural irregularity 13
Myoglobin, structure 384
Myoglobin, structure, computer-generated schematic diagram 22F
Myoglobin, structure, early results 13 13F
Myoglobin, structure, schematic diagram 23F
Myoglobin, structure, two-dimensional 22F
Myoglobin, x-ray diffraction 379
Myohemerythrin 37 381F
myosin 36 197 256 290Ч291 291F
Myosin, actin complex, structure 295 295F
Myosin, conformational change 294Ч295 296
Myosin, cross-bridge movement 291Ч292 295
Myosin, cross-bridge movement, confirmation 292Ч293 295Ч296
Myosin, nucleotide-binding cleft 295 296
Myosin, S1 fragment 294 294F 295
Myosin, sliding filament model 291 291F
Myosin, structure 292 294Ч295 294F
Myosin, swinging cross-bridge hypothesis 292 292F 295Ч296 296F
Nef protein 275 275F 276F
Neuraminidase 70Ч71
Neuraminidase, active site 71F 72
Neuraminidase, amino acids 71
Neuraminidase, folding motifs in propeller-like structure 71Ч72 71F 73F
Neuraminidase, function 70Ч71
Neuraminidase, subunit structure 71 71F 72F
Neurofilament proteins 287F
Neurospora crassa, PRA isomerase and IGP synthase 53
Neutrofil elastase 110
NF-kB 168Ч169
NMR 374 387Ч388
NMR, advantages 391
NMR, COSY 388 388F 389
NMR, distance constraints 390Ч391
NMR, folded protein flexibility 105
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 sheets
sheets  -horseshoe fold
-horseshoe fold  helix
helix 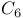 -zinc cluster family binding to DNA
-zinc cluster family binding to DNA 
 motif
motif 
 gene, homeodomain
gene, homeodomain