|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Miessler G., Tarr D.A. — Inorganic Chemistry |
|
|
 |
| Предметный указатель |
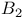 , molecular orbitals 127 , molecular orbitals 127
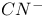 (cyanide), as ligand 475 (cyanide), as ligand 475
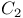 axes, perpendicular 86 87 101 axes, perpendicular 86 87 101
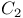 , molecular orbitals 127 , molecular orbitals 127
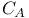 , , 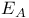 , ,  , and , and 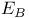 values 190 values 190
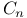 axis 82 axis 82
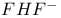 , hydrogen bonding 174 , hydrogen bonding 174
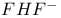 , molecular orbitals 140—143 175 , molecular orbitals 140—143 175
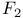 , molecular orbitals 127 128 , molecular orbitals 127 128
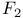 , symmetry 90 , symmetry 90
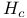 , critical magnetic field for superconductivity 228 , critical magnetic field for superconductivity 228
 53 53
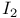 , spectra in different solvents 178 , spectra in different solvents 178
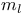 , magnetic quantum number 26 , magnetic quantum number 26
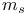 , spin quantum number 26 27 , spin quantum number 26 27
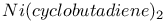 , symmetry 90 , symmetry 90
 (cyanate ion), VSEPR and structure 54 (cyanate ion), VSEPR and structure 54
 (peroxynitrite), structure 278 (peroxynitrite), structure 278
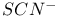 (thiocyanate) VSEPR and structure 54 (thiocyanate) VSEPR and structure 54
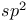 hybrids in water 158 hybrids in water 158
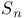 , rotation-reflection operation 80 , rotation-reflection operation 80
 CB mechanism 426 427 CB mechanism 426 427
 lim mechanism 416 lim mechanism 416
 lim mechanism 416 lim mechanism 416
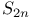 , axes 83 88 , axes 83 88
![$[Co(tren)(sal)]^+$](/math_tex/565f1a38dc73cf91922f4f04dcfeaa0782.gif) isomers 313 isomers 313
 decay 8 decay 8
 from temperature dependence of equilibrium constant 192 193 from temperature dependence of equilibrium constant 192 193
 from temperature dependence of equilibrium constant 193 from temperature dependence of equilibrium constant 193
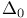 in octahedral complexes 346 in octahedral complexes 346
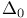 in octahedral complexes, determining from spectra 393—395 401 402 in octahedral complexes, determining from spectra 393—395 401 402
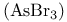 , bond angle 66 , bond angle 66
 , bond angle 66 , bond angle 66
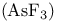 , bond angle 66 , bond angle 66
 , bond angle 66 , bond angle 66
 , bond angle, VSEPR and structure 66 , bond angle, VSEPR and structure 66
 , ferrocene 457 , ferrocene 457
 structure 296 structure 296
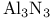 ring 261 ring 261
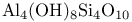 (kaolinite) 234 (kaolinite) 234
 fragment 566 fragment 566
 , symmetry 89 , symmetry 89
 , bonding 56 57 , bonding 56 57
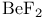 , bonding 56 57 , bonding 56 57
 , molecular orbitals 127 , molecular orbitals 127
 adduct 170 171 adduct 170 171
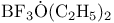 adduct, boiling point 171 adduct, boiling point 171
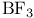 , bonding 58 59 , bonding 58 59
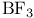 , molecular orbitals 154—156 , molecular orbitals 154—156
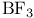 , symmetry 89 , symmetry 89
 290 290
 , solvent 168 , solvent 168
 , VSEPR and structure 60 , VSEPR and structure 60
 290 290
 , symmetry 89 , symmetry 89
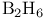 , bonding 256—258 , bonding 256—258
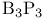 rings 261 rings 261
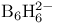 , bonding 573 574 , bonding 573 574
 , symmetry 85 86 , symmetry 85 86
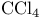 , dipole moment 68 , dipole moment 68
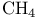 , dipole moment 68 , dipole moment 68
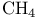 , symmetry 85 86 , symmetry 85 86
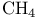 , VSEPR and structure 59 , VSEPR and structure 59
 53 290 53 290
 , VSEPR and structure 60 , VSEPR and structure 60
 290 290
 , structure 62 , structure 62
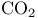 (carbon dioxide) 267 (carbon dioxide) 267
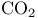 (carbon dioxide) and greenhouse effect 634 (carbon dioxide) and greenhouse effect 634
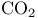 (carbon dioxide), electron-dot diagram 52 (carbon dioxide), electron-dot diagram 52
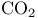 (carbon dioxide), geometry 52 (carbon dioxide), geometry 52
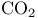 (carbon dioxide), molecular orbitals 143—147 (carbon dioxide), molecular orbitals 143—147
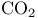 (carbon dioxide), symmetry 85 86 (carbon dioxide), symmetry 85 86
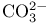 (carbonate ion), electron-dot diagram 52 (carbonate ion), electron-dot diagram 52
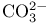 (carbonate ion), molecular orbitals 156 (carbonate ion), molecular orbitals 156
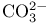 (carbonate ion), structure and dipole moment 68 (carbonate ion), structure and dipole moment 68
![$\mathrm{Cr(CO)_5[C(OCH_3)C_6H_5]}$](/math_tex/f8d00a1d2a8de7ba9405c552c095391e82.gif) 499 500 499 500
![$\mathrm{Cr(CO)_5[C(OCH_3)C_6H_5]}$](/math_tex/f8d00a1d2a8de7ba9405c552c095391e82.gif) , , 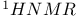 499 499
![$\mathrm{Cr(CO)_5[C(OCH_3)C_6H_5]}$](/math_tex/f8d00a1d2a8de7ba9405c552c095391e82.gif) , cis and trans isomers 499 500 , cis and trans isomers 499 500
![$\mathrm{Cr(CO)_5[C(OCH_3)C_6H_5]}$](/math_tex/f8d00a1d2a8de7ba9405c552c095391e82.gif) , synthesis 499 , synthesis 499
 and 18—electron rule 463—465 and 18—electron rule 463—465
 , molecular orbitals 464 , molecular orbitals 464
 , symmetry 85 , symmetry 85
 90 90
 , electron-dot diagram and geometry 52 , electron-dot diagram and geometry 52
 and CO complexes 491 and CO complexes 491
 , cyclopentadienyl 485 , cyclopentadienyl 485
 synthesis 535 synthesis 535
 , buckminsterfullerene 4 265 , buckminsterfullerene 4 265
 , buckminsterfullerene as ligand 493—495 , buckminsterfullerene as ligand 493—495
 265 265
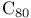 265 265
 reactions 443 reactions 443
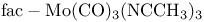 , CO stretching modes 110 , CO stretching modes 110
 (staggered), symmetry 90 (staggered), symmetry 90
 495 495
 as acid 178 197 198 as acid 178 197 198
 , halide charge-transfer complexes 179 , halide charge-transfer complexes 179
 287 287
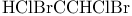 , symmetry 84 , symmetry 84
 287 287
 , symmetry 85 86 , symmetry 85 86
$\mathrm{HCN}, bonding 62
$\mathrm{HCN}, symmetry 89
 527 527
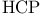 62 62
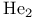 , molecular orbitals 126 , molecular orbitals 126
 , hydroformylation catalyst 537 538 , hydroformylation catalyst 537 538
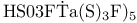 204 204
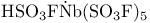 204 204
 , acid strength 197 , acid strength 197
 , symmetry 84 , symmetry 84
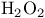 , symmetry 87 88 , symmetry 87 88
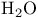 , bond angle 60 66 , bond angle 60 66
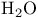 , symmetry 82 89 , symmetry 82 89
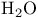 , VSEPR and structure 59 66 , VSEPR and structure 59 66
 , VSEPR and structure 66 , VSEPR and structure 66
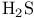 VSEPR and structure 66 VSEPR and structure 66
 , VSEPR and structure 66 , VSEPR and structure 66
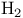 , bonding 118 , bonding 118
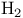 , complexes 478 , complexes 478
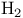 , molecular orbitals 125 , molecular orbitals 125
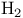 , source 275 , source 275
 , symmetry 87 88 , symmetry 87 88
 , acid strength 197 , acid strength 197
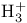 ion, molecular orbitals 143 ion, molecular orbitals 143
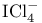 290 290
 , symmetry 90 , symmetry 90
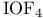 62 62
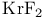 295 295
![$\mathrm{K[Pt(C_2H_4)Cl_3]\dot H_2O}$](/math_tex/e155c9bb98d7562a0c9d47ea995ff49a82.gif) , Zeise’s salt 457 482 483 , Zeise’s salt 457 482 483
 248 248
 , molecular orbitals 127 , molecular orbitals 127
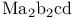 isomers 314 isomers 314
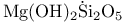 Minerals, structure 235 Minerals, structure 235
 234 234
 , radius ratio 219 , radius ratio 219
 , structure 215 , structure 215
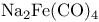 (Collman’s reagent) 527 (Collman’s reagent) 527
 , bond angle 66 , bond angle 66
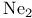 , molecular orbitals 127 129 , molecular orbitals 127 129
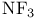 , bond angle 66 , bond angle 66
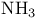 87 88 87 88
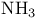 , character table 99 , character table 99
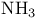 , molecular orbitals 151—153 , molecular orbitals 151—153
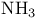 , symmetry 87 88 99 , symmetry 87 88 99
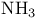 , synthesis 274 , synthesis 274
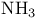 , VSEPR and structure 60 61 66 68 , VSEPR and structure 60 61 66 68
 , molecular energy levels 172 , molecular energy levels 172
 457 457
|  , molecular orbitals 360 361 , molecular orbitals 360 361
 , synthesis 473 , synthesis 473
 crystal structure 217 crystal structure 217
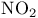 , nitrogen dioxide 276 , nitrogen dioxide 276
 (nitrate ion), molecular orbitals 156 (nitrate ion), molecular orbitals 156
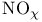 and acid rain 276 and acid rain 276
 (nitrosyl), as ligand 476 (nitrosyl), as ligand 476
 (nitrosyl), as ligand, complexes 476 (nitrosyl), as ligand, complexes 476
 (nitrosyl), as ligand, linear and bent bonding modes 476 (nitrosyl), as ligand, linear and bent bonding modes 476
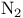 complexes 475 complexes 475
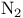 , molecular orbitals 128 , molecular orbitals 128
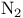 , photoelectron spectrum and molecular orbitals 131 , photoelectron spectrum and molecular orbitals 131
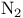 , symmetry 90 , symmetry 90
 , , symmetry 89 , , symmetry 89
 , , product of catalytic converters 628 , , product of catalytic converters 628
 , structure , structure
 , , molecular orbital diagram 147 , , molecular orbital diagram 147
 , synthesis and structure 272 , synthesis and structure 272
 , bond angle 66 , bond angle 66
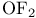 , bond angle 66 , bond angle 66
 (eclipsed), symmetry 89 (eclipsed), symmetry 89
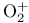 (dioxygenyl ion) 128 (dioxygenyl ion) 128
 (superoxide ion) 128 (superoxide ion) 128
 (peroxide ion) 128 (peroxide ion) 128
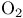 (dioxygen) 128 (dioxygen) 128
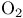 (dioxygen), molecular orbitals 127 128 (dioxygen), molecular orbitals 127 128
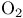 (dioxygen), paramagnetic 128 280 (dioxygen), paramagnetic 128 280
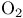 (dioxygen), photoelectron spectrum and molecular orbitals 131 (dioxygen), photoelectron spectrum and molecular orbitals 131
 , symmetry 89 , symmetry 89
 , bond angle 66 , bond angle 66
 , bond angle 66 , bond angle 66
 , symmetry 89 , symmetry 89
 , structure 62 , structure 62
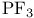 , bond angle 66 , bond angle 66
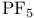 , symmetry 87 , symmetry 87
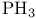 , VSEPR and structure 66 , VSEPR and structure 66
 55 55
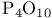 , and phosphoric acid synthesis 279 , and phosphoric acid synthesis 279
 , electronic equivalents 557 , electronic equivalents 557
 , structure 273 , structure 273
 , Wilkinson’s catalyst 542 , Wilkinson’s catalyst 542
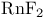 295 295
 495 495
 , bond angle 66 , bond angle 66
 , bond angle 66 , bond angle 66
 , bond angle 66 , bond angle 66
 62 62
 as acid 168 as acid 168
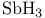 , VSEPR and structure 66 , VSEPR and structure 66
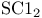 , bond angle 66 , bond angle 66
 496 496
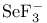 62 62
 62 62
 , bond angle 66 , bond angle 66
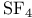 , symmetry 90 , symmetry 90
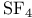 , VSEPR and structure 60 61 , VSEPR and structure 60 61
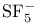 62 62
 and natural orbital bonding 161 and natural orbital bonding 161
 , structure 53 58 , structure 53 58
 , symmetry 85 86 , symmetry 85 86
 , structure 62 , structure 62
 55 55
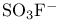 55 55
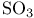 , dipole moment 68 , dipole moment 68
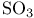 , electron-dot structure 52 56 , electron-dot structure 52 56
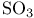 , hybrid orbitals, , hybrid orbitals, 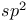 158 158
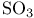 , molecular orbitals 156 , molecular orbitals 156
 17-electron 17-electron
 compounds 292 compounds 292
 , structure 294 , structure 294
 , structure 293 , structure 293
 , synthesis 293 , synthesis 293
 , VSEPR and structure 61 , VSEPR and structure 61
 , structure 293 , structure 293
 , symmetry 90 , symmetry 90
 , synthesis 293 , synthesis 293
 , structure 294 , structure 294
 , structure 62 , structure 62
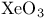 reactions 295 reactions 295
 reactions 295 reactions 295
 , structure 230 , structure 230
 , superconductor 230 , superconductor 230
![$\mathrm{[(CH_3)A1N}(2, 6—diisopropylphenyl)]_3$](/math_tex/e48ac36c71fe29bbce9b1faf99c3480682.gif) 261 261
![$\mathrm{[(\eta^5-C_5H_5)Mo(CO)_2]_2}$](/math_tex/8cac1625a16e1cd8005b6affd3a1171e82.gif) and bridging carbonyls 471 and bridging carbonyls 471
![$\mathrm{[Co(Co(NH_3)_4(OH)_2)_3]Br_6}$](/math_tex/164318b9df0147fa7277d149511ebb8c82.gif) , totally inorganic optically active compound 301 , totally inorganic optically active compound 301
![$\mathrm{[Co(CO)_4]^-}$](/math_tex/6d5038f3e2e004d9f12c041670f8214682.gif) 527 527
![$\mathrm{[Co(en)_2(H_2O)X]^{n+}}$](/math_tex/bc5f842e8361168f68b0662ff1d4ecde82.gif) , rates of substitution reactions 432 , rates of substitution reactions 432
![$\mathrm{[Co(en)_3]^{3+}}$](/math_tex/80ff32c8a7672663ac81e16efc47632782.gif) , chirality of ring conformation 318 , chirality of ring conformation 318
![$\mathrm{[Co(en)_3]^{3+}}$](/math_tex/80ff32c8a7672663ac81e16efc47632782.gif) , symmetry 87 88 , symmetry 87 88
![$\mathrm{[Co(H_2NC_2H_4NH_2)_2Cl_2]^+}$](/math_tex/5543822206eb28363123cdd156df918682.gif) , cis and trans isomers 301 , cis and trans isomers 301
![$\mathrm{[Co(NH_3)_4Cl_2]^+}$](/math_tex/e0544f8bb19c6041a41f4cbc455c305782.gif) , cis and trans isomers 301 , cis and trans isomers 301
![$\mathrm{[Co(NH_3)_5(H_2O)]^{3+}}$](/math_tex/49d88c6d2b24736ed05b6f618f1c298c82.gif) , rates of substitution 424 , rates of substitution 424
![$\mathrm{[CoX_2(trien)]^+}$](/math_tex/afc862274ad281baa594b57c1fcf79b382.gif) , ,  and and  forms 319 forms 319
![$\mathrm{[Cu(H_2O)_6]^{2+}}$](/math_tex/2d0cdfe9966f4d694a230102924b231682.gif) absorption spectrum 399 absorption spectrum 399
![$\mathrm{[Cu(H_2O)_6]^{2+}}$](/math_tex/2d0cdfe9966f4d694a230102924b231682.gif) absorption spectrum, color 380 absorption spectrum, color 380
![$\mathrm{[Fe(CN)_5(NO)]^{2-}}$](/math_tex/f9763f0723782c01536c76cd42f9e84582.gif) , vasodilator 477 , vasodilator 477
![$\mathrm{[Fe(CO)_2(CN)_4]^{2-}}$](/math_tex/30493e4046ba78bb2d11685849d9e6ca82.gif) 475 475
![$\mathrm{[Fe(CO)_3(CN)_3]^-}$](/math_tex/d85ea95c0b941332bf5f5e2abaecb3e382.gif) 475 475
![$\mathrm{[Fe(CO)_]^{2-}}$](/math_tex/08cbb4767e33bb3a233dbfe59d8ac29182.gif) , in synthesis 527 , in synthesis 527
![$\mathrm{[Fe(trien)]^{3+}}$](/math_tex/2bbe7f25a5e2c5345078536e9618fdd182.gif) , peroxide decomposition, catalyst 600 , peroxide decomposition, catalyst 600
![$\mathrm{[M(H_2O)_6]^{n+}}$](/math_tex/231e6a646900e1a2cad2dc9aab2c37d882.gif) , absorption spectra 397 , absorption spectra 397
![$\mathrm{[Mn(H_2O)_6]^{2+}}$](/math_tex/3de40a6b01d0622e9f8f33cea0d1c1e282.gif) , absorption spectrum 397 405 , absorption spectrum 397 405
![$\mathrm{[Mo_2Cl_8]^{4-}}$](/math_tex/d06b4cfe7f99c817cc33e830a2c84ffc82.gif) , spectrum and bonding 570 , spectrum and bonding 570
![$\mathrm{[Ni(H_2O)_6]^{2+}}$](/math_tex/aeb2f22390b9edf81a961ba94a4f604b82.gif) , rates of substitution 424 , rates of substitution 424
![$\mathrm{[Os_2Cl_8]^{2-}}$](/math_tex/35dd37399a6a35f401dd766be79ef38182.gif) , bonding 570 , bonding 570
![$\mathrm{[Pt(Br)(Cl)(I)(NH_3)(NO_2)(py)]}$](/math_tex/1a8f5b1e8d4f802fc3a862b6505554ca82.gif) isomers 313 isomers 313
![$\mathrm{[Pt(CN)_4]^{2-}}$](/math_tex/ce949da09238066c6bc2f53443cdb4ca82.gif) ion, bonding 358 ion, bonding 358
![$\mathrm{[Pt(CN)_4]^{2-}}$](/math_tex/ce949da09238066c6bc2f53443cdb4ca82.gif) ion, spectra and ligand field splitting 360 ion, spectra and ligand field splitting 360
![$\mathrm{[PtCl_4]^{2-}}$](/math_tex/f87d9c42ec73889987513ab06cfd563b82.gif) , spectrum and ligand field splitting 360 , spectrum and ligand field splitting 360
![$\mathrm{[PtCl_4]^{2-}}$](/math_tex/f87d9c42ec73889987513ab06cfd563b82.gif) , symmetry 90 , symmetry 90
![$\mathrm{[Re_2Cl_8]^{2-}}$](/math_tex/290da515c7baa2086be3f46ed9532b8682.gif) , , 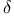 bond 569 bond 569
![$\mathrm{[Re_2Cl_8]^{2-}}$](/math_tex/290da515c7baa2086be3f46ed9532b8682.gif) , bonding 569 , bonding 569
![$\mathrm{[Re_2Cl_8]^{2-}}$](/math_tex/290da515c7baa2086be3f46ed9532b8682.gif) , spectrum and bonding 570 , spectrum and bonding 570
![$\mathrm{[Ru(II)(EDTA)(H_2O)]^{2-}}$](/math_tex/561ce37b1fa6540968b484a27008d0c082.gif) , rates of substitution reactions 426 , rates of substitution reactions 426
![$\mathrm{[Ru(III)(EDTA)(H_2O)]^-}$](/math_tex/1f1d399b70f6248c45ffac053dd09f3782.gif) , rates of substitution reactions 426 , rates of substitution reactions 426
![$\mathrm{[Ru(NH_2CH_2CH_2NH_2)_3]^{2+}}$](/math_tex/b9dc09d23160b79cb0a8237432a62bab82.gif) , symmetry 90 , symmetry 90
![$\mathrm{[TaF_8]^{3-}}$](/math_tex/bd88f38f5685471f05f477d2ae09cd3382.gif) , VSEPR and structure 53 58 , VSEPR and structure 53 58
![$\mathrm{[Ti(H_2O)_6]^{3+}}$](/math_tex/58914bd10476f0494b73bcbe12d07f2b82.gif) , absorption spectrum 400 , absorption spectrum 400
![$\mathrm{[V(H_2O)_6]^{3+}}$](/math_tex/c599d1d09f8b9a4653b29c18cb0ca5b582.gif) , absorption spectrum 394 , absorption spectrum 394
![$\mathrm{[XeF_8]^{2-}}$](/math_tex/2040c467c62e2a991f14598b131a701d82.gif) , VSEPR and structure 53 , VSEPR and structure 53
![$\mathrm{[XeF_8]^{2-}}$](/math_tex/2040c467c62e2a991f14598b131a701d82.gif) , VSEPR and structure, structure 294 , VSEPR and structure, structure 294
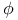 , angular function 28 , angular function 28
 acceptor ligands 364 365 368 acceptor ligands 364 365 368
 acceptor ligands of acceptor ligands of 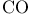 463 467—470 463 467—470
 acceptor ligands, angular overlap 364 365 acceptor ligands, angular overlap 364 365
 acceptor ligands, back-bonding 354 355 367 368 acceptor ligands, back-bonding 354 355 367 368
 bonding and bonding and 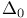 355 355
 bonding and LFSE 355 bonding and LFSE 355
 bonding in carbene complexes 499 bonding in carbene complexes 499
 bonding, octahedral complexes 352—355 bonding, octahedral complexes 352—355
 bonding, orbital overlap in octahedral complexes 354 bonding, orbital overlap in octahedral complexes 354
 bonding, orbitals, square planar complexes 357 bonding, orbitals, square planar complexes 357
 donor ligands 366—368 donor ligands 366—368
 donor ligands, angular overlap 366 donor ligands, angular overlap 366
 interactions, between interactions, between 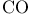 and a metal 468 and a metal 468
 orbitals from d orbitals 120 orbitals from d orbitals 120
 orbitals from p orbitals 119 120 orbitals from p orbitals 119 120
 orbitals from p orbitals and group theory 134 orbitals from p orbitals and group theory 134
 -Allyl complexes 483 484 -Allyl complexes 483 484
 -allyl radical, as ligand 479 -allyl radical, as ligand 479
 -bonded aromatic rings 3 4 -bonded aromatic rings 3 4
 -ethylene complexes 482—484 -ethylene complexes 482—484
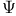 , wave function 21 22 116 , wave function 21 22 116
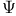 , wave function, properties 22 23 , wave function, properties 22 23
 donor basicity 367 donor basicity 367
 interactions, between CO and a metal 468 interactions, between CO and a metal 468
 orbitals 117 118 120 122 134 orbitals 117 118 120 122 134
 orbitals from d orbitals 120 orbitals from d orbitals 120
 orbitals from p orbitals 120 orbitals from p orbitals 120
 orbitals, square planar complexes 356 orbitals, square planar complexes 356
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



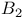 , molecular orbitals
, molecular orbitals 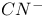 (cyanide), as ligand
(cyanide), as ligand 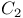 axes, perpendicular
axes, perpendicular 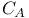 ,
, 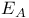 ,
,  , and
, and 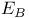 values
values 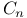 axis
axis 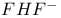 , hydrogen bonding
, hydrogen bonding 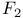 , molecular orbitals
, molecular orbitals 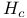 , critical magnetic field for superconductivity
, critical magnetic field for superconductivity 
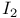 , spectra in different solvents
, spectra in different solvents 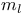 , magnetic quantum number
, magnetic quantum number 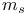 , spin quantum number
, spin quantum number 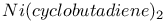 , symmetry
, symmetry  (cyanate ion), VSEPR and structure
(cyanate ion), VSEPR and structure  (peroxynitrite), structure
(peroxynitrite), structure 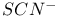 (thiocyanate) VSEPR and structure
(thiocyanate) VSEPR and structure 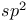 hybrids in water
hybrids in water 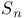 , rotation-reflection operation
, rotation-reflection operation  CB mechanism
CB mechanism  lim mechanism
lim mechanism 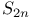 , axes
, axes ![$[Co(tren)(sal)]^+$](/math_tex/565f1a38dc73cf91922f4f04dcfeaa0782.gif) isomers
isomers  decay
decay  from temperature dependence of equilibrium constant
from temperature dependence of equilibrium constant  from temperature dependence of equilibrium constant
from temperature dependence of equilibrium constant 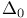 in octahedral complexes
in octahedral complexes 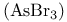 , bond angle
, bond angle  , bond angle
, bond angle 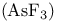 , bond angle
, bond angle  , bond angle
, bond angle  , ferrocene
, ferrocene  structure
structure 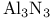 ring
ring 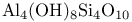 (kaolinite)
(kaolinite)  fragment
fragment  , symmetry
, symmetry  , bonding
, bonding 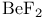 , bonding
, bonding  , molecular orbitals
, molecular orbitals  adduct
adduct 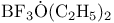 adduct, boiling point
adduct, boiling point 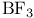 , bonding
, bonding 

 , symmetry
, symmetry 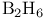 , bonding
, bonding 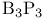 rings
rings 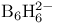 , bonding
, bonding  , symmetry
, symmetry 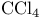 , dipole moment
, dipole moment 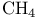 , dipole moment
, dipole moment 

 , structure
, structure 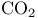 (carbon dioxide)
(carbon dioxide) 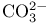 (carbonate ion), electron-dot diagram
(carbonate ion), electron-dot diagram ![$\mathrm{Cr(CO)_5[C(OCH_3)C_6H_5]}$](/math_tex/f8d00a1d2a8de7ba9405c552c095391e82.gif)
 and 18—electron rule
and 18—electron rule  , symmetry
, symmetry 
 and CO complexes
and CO complexes  synthesis
synthesis  , buckminsterfullerene
, buckminsterfullerene 
 reactions
reactions 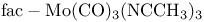 , CO stretching modes
, CO stretching modes  (staggered), symmetry
(staggered), symmetry 
 as acid
as acid 
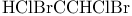 , symmetry
, symmetry 

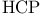
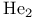 , molecular orbitals
, molecular orbitals  , hydroformylation catalyst
, hydroformylation catalyst 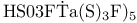
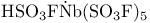
 , acid strength
, acid strength  , symmetry
, symmetry 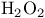 , symmetry
, symmetry 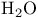 , bond angle
, bond angle  , VSEPR and structure
, VSEPR and structure 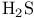 VSEPR and structure
VSEPR and structure  , VSEPR and structure
, VSEPR and structure 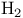 , bonding
, bonding  , symmetry
, symmetry  , acid strength
, acid strength 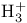 ion, molecular orbitals
ion, molecular orbitals 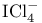
 , symmetry
, symmetry 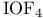
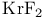
![$\mathrm{K[Pt(C_2H_4)Cl_3]\dot H_2O}$](/math_tex/e155c9bb98d7562a0c9d47ea995ff49a82.gif) , Zeise’s salt
, Zeise’s salt 
 , molecular orbitals
, molecular orbitals 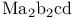 isomers
isomers 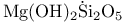 Minerals, structure
Minerals, structure 
 , radius ratio
, radius ratio 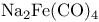 (Collman’s reagent)
(Collman’s reagent)  , bond angle
, bond angle 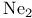 , molecular orbitals
, molecular orbitals 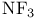 , bond angle
, bond angle 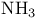
 , molecular energy levels
, molecular energy levels 
 crystal structure
crystal structure 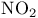 , nitrogen dioxide
, nitrogen dioxide  (nitrate ion), molecular orbitals
(nitrate ion), molecular orbitals 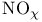 and acid rain
and acid rain  (nitrosyl), as ligand
(nitrosyl), as ligand 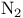 complexes
complexes  , , symmetry
, , symmetry  , , product of catalytic converters
, , product of catalytic converters  ,
,  , , molecular orbital diagram
, , molecular orbital diagram  , synthesis and structure
, synthesis and structure  , bond angle
, bond angle 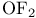 , bond angle
, bond angle  (eclipsed), symmetry
(eclipsed), symmetry 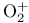 (dioxygenyl ion)
(dioxygenyl ion)  (superoxide ion)
(superoxide ion)  (peroxide ion)
(peroxide ion) 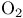 (dioxygen)
(dioxygen)  , symmetry
, symmetry  , bond angle
, bond angle  , bond angle
, bond angle  , structure
, structure 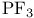 , bond angle
, bond angle 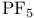 , symmetry
, symmetry 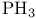 , VSEPR and structure
, VSEPR and structure 
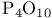 , and phosphoric acid synthesis
, and phosphoric acid synthesis  , electronic equivalents
, electronic equivalents  , Wilkinson’s catalyst
, Wilkinson’s catalyst 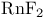

 , bond angle
, bond angle  , bond angle
, bond angle  , bond angle
, bond angle 
 as acid
as acid 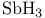 , VSEPR and structure
, VSEPR and structure 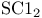 , bond angle
, bond angle 
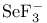

 , bond angle
, bond angle 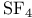 , symmetry
, symmetry 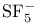
 and natural orbital bonding
and natural orbital bonding  , structure
, structure 
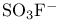
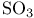 , dipole moment
, dipole moment 
 compounds
compounds  , structure
, structure  , structure
, structure  , structure
, structure  , structure
, structure  , structure
, structure 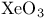 reactions
reactions  reactions
reactions  , structure
, structure ![$\mathrm{[(CH_3)A1N}(2, 6—diisopropylphenyl)]_3$](/math_tex/e48ac36c71fe29bbce9b1faf99c3480682.gif)
![$\mathrm{[(\eta^5-C_5H_5)Mo(CO)_2]_2}$](/math_tex/8cac1625a16e1cd8005b6affd3a1171e82.gif) and bridging carbonyls
and bridging carbonyls ![$\mathrm{[Co(Co(NH_3)_4(OH)_2)_3]Br_6}$](/math_tex/164318b9df0147fa7277d149511ebb8c82.gif) , totally inorganic optically active compound
, totally inorganic optically active compound ![$\mathrm{[Co(CO)_4]^-}$](/math_tex/6d5038f3e2e004d9f12c041670f8214682.gif)
![$\mathrm{[Co(en)_2(H_2O)X]^{n+}}$](/math_tex/bc5f842e8361168f68b0662ff1d4ecde82.gif) , rates of substitution reactions
, rates of substitution reactions ![$\mathrm{[Co(en)_3]^{3+}}$](/math_tex/80ff32c8a7672663ac81e16efc47632782.gif) , chirality of ring conformation
, chirality of ring conformation ![$\mathrm{[Co(H_2NC_2H_4NH_2)_2Cl_2]^+}$](/math_tex/5543822206eb28363123cdd156df918682.gif) , cis and trans isomers
, cis and trans isomers ![$\mathrm{[Co(NH_3)_4Cl_2]^+}$](/math_tex/e0544f8bb19c6041a41f4cbc455c305782.gif) , cis and trans isomers
, cis and trans isomers ![$\mathrm{[Co(NH_3)_5(H_2O)]^{3+}}$](/math_tex/49d88c6d2b24736ed05b6f618f1c298c82.gif) , rates of substitution
, rates of substitution ![$\mathrm{[CoX_2(trien)]^+}$](/math_tex/afc862274ad281baa594b57c1fcf79b382.gif) ,
,  and
and ![$\mathrm{[Cu(H_2O)_6]^{2+}}$](/math_tex/2d0cdfe9966f4d694a230102924b231682.gif) absorption spectrum
absorption spectrum ![$\mathrm{[Fe(CN)_5(NO)]^{2-}}$](/math_tex/f9763f0723782c01536c76cd42f9e84582.gif) , vasodilator
, vasodilator ![$\mathrm{[Fe(CO)_2(CN)_4]^{2-}}$](/math_tex/30493e4046ba78bb2d11685849d9e6ca82.gif)
![$\mathrm{[Fe(CO)_3(CN)_3]^-}$](/math_tex/d85ea95c0b941332bf5f5e2abaecb3e382.gif)
![$\mathrm{[Fe(CO)_]^{2-}}$](/math_tex/08cbb4767e33bb3a233dbfe59d8ac29182.gif) , in synthesis
, in synthesis ![$\mathrm{[Fe(trien)]^{3+}}$](/math_tex/2bbe7f25a5e2c5345078536e9618fdd182.gif) , peroxide decomposition, catalyst
, peroxide decomposition, catalyst ![$\mathrm{[M(H_2O)_6]^{n+}}$](/math_tex/231e6a646900e1a2cad2dc9aab2c37d882.gif) , absorption spectra
, absorption spectra ![$\mathrm{[Mn(H_2O)_6]^{2+}}$](/math_tex/3de40a6b01d0622e9f8f33cea0d1c1e282.gif) , absorption spectrum
, absorption spectrum ![$\mathrm{[Mo_2Cl_8]^{4-}}$](/math_tex/d06b4cfe7f99c817cc33e830a2c84ffc82.gif) , spectrum and bonding
, spectrum and bonding ![$\mathrm{[Ni(H_2O)_6]^{2+}}$](/math_tex/aeb2f22390b9edf81a961ba94a4f604b82.gif) , rates of substitution
, rates of substitution ![$\mathrm{[Os_2Cl_8]^{2-}}$](/math_tex/35dd37399a6a35f401dd766be79ef38182.gif) , bonding
, bonding ![$\mathrm{[Pt(Br)(Cl)(I)(NH_3)(NO_2)(py)]}$](/math_tex/1a8f5b1e8d4f802fc3a862b6505554ca82.gif) isomers
isomers ![$\mathrm{[Pt(CN)_4]^{2-}}$](/math_tex/ce949da09238066c6bc2f53443cdb4ca82.gif) ion, bonding
ion, bonding ![$\mathrm{[PtCl_4]^{2-}}$](/math_tex/f87d9c42ec73889987513ab06cfd563b82.gif) , spectrum and ligand field splitting
, spectrum and ligand field splitting ![$\mathrm{[Re_2Cl_8]^{2-}}$](/math_tex/290da515c7baa2086be3f46ed9532b8682.gif) ,
, 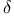 bond
bond ![$\mathrm{[Ru(II)(EDTA)(H_2O)]^{2-}}$](/math_tex/561ce37b1fa6540968b484a27008d0c082.gif) , rates of substitution reactions
, rates of substitution reactions ![$\mathrm{[Ru(III)(EDTA)(H_2O)]^-}$](/math_tex/1f1d399b70f6248c45ffac053dd09f3782.gif) , rates of substitution reactions
, rates of substitution reactions ![$\mathrm{[Ru(NH_2CH_2CH_2NH_2)_3]^{2+}}$](/math_tex/b9dc09d23160b79cb0a8237432a62bab82.gif) , symmetry
, symmetry ![$\mathrm{[TaF_8]^{3-}}$](/math_tex/bd88f38f5685471f05f477d2ae09cd3382.gif) , VSEPR and structure
, VSEPR and structure ![$\mathrm{[Ti(H_2O)_6]^{3+}}$](/math_tex/58914bd10476f0494b73bcbe12d07f2b82.gif) , absorption spectrum
, absorption spectrum ![$\mathrm{[V(H_2O)_6]^{3+}}$](/math_tex/c599d1d09f8b9a4653b29c18cb0ca5b582.gif) , absorption spectrum
, absorption spectrum ![$\mathrm{[XeF_8]^{2-}}$](/math_tex/2040c467c62e2a991f14598b131a701d82.gif) , VSEPR and structure
, VSEPR and structure  , angular function
, angular function  acceptor ligands
acceptor ligands 
 , wave function
, wave function  donor basicity
donor basicity