|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Miessler G., Tarr D.A. — Inorganic Chemistry |
|
|
 |
| Предметный указатель |
Difluorodiazene, symmetry 89
Dihydrogen  as ligand 456 as ligand 456
Dihydrogen  , complexes, bonding 477 478 , complexes, bonding 477 478
Dimension of a representation 98
Dimers 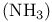 70 70
Diodes, behavior 226
diodes, light-emitting 226 227
Diodes, photovoltaic cells 226
Diodes, structure 226
Dioldehydrase 595
Dioxovanadium 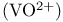 , acidity 198 , acidity 198
Dioxygen  128 281 128 281
Dioxygenyl ion 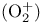 128 281 128 281
Dipeptidase 595
Dipole moment 67 68
Dislocations in crystals 213 232
Dispersion forces 69
Displacement, nucleophilic 526 527
Dissociation energy, Born — Haber cycle 138
Dissociative interchange, 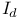 415 415
Dissociative mechanism (D) 417 418 420—422
Dissociative mechanism (D), evidence for 422
Dissociative mechanism (D), phosphine, mechanism 521—523
Dissociative mechanism (D), rate equation 417—419
Dissociative mechanism (D), Ru(II) compounds 425
Dissociative mechanism (D), stereochemical changes for cis-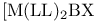 433 433
Dissymmetric 102
Distorted T geometry, VSEPR 61
DMSO reductase 595
DNA polymerase 595
DNA polymerase, mechanism 623
DNA, cleavage studies 622 624 625
DNA, double helix 620
DNA, structure 619
Dodecahedral geometry 332 333
Donor-acceptor bonding in 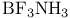 170 170
Donor-acceptor transition 178
Doped semiconductors 224
Drago, R.S. 189—191
E, C parameters 189—191 675
Earnshaw, A. 14 237
Earth, formation 5 8
Earth, structure 9
EDTA complex, handedness of rings 316
Effective nuclear charge (Z*) 38 40 41
Effects of entering group and cis-ligands on rates 425
Eighteen-electron rule 304 460 462 463 465
Eighteen-electron rule, exceptions 465
Electrical resistivity and metallic character 241
Electrides 252
Electrode potentials 245 246 278 288
Electron 5 6
Electron affinity 44 139 672 673
Electron affinity, Born — Haber cycle 220
Electron configurations of the elements 39
Electron configurations, transition elements 40—42
Electron counting in cluster compounds 583
Electron counting in organometallic compounds 459 460 462 463 465
Electron counting in square planar complexes 465 466
Electron counting, common ligands 462
Electron density 21
Electron spin 340
Electron-dot diagrams and formal charge 691—695
Electron-electron interactions in transition metal atoms 38 41
Electron-pair acceptor 170
Electron-pair donor 170
Electronegativity 63 65 66 243 673
Electronegativity and bond energies 64 65
Electronegativity and bond polarity 65 66 68
Electronegativity and VSEPR 65 66
Electronegativity, absolute (x) 187 189
Electronegativity, Allen, L.C 64
Electronegativity, Allred, A.L. 64
Electronegativity, and acidity or basicity 195
Electronegativity, Jaffe, H.H. 64
Electronegativity, Mulliken, R.S. 64 187
Electronegativity, noble gases 243
Electronegativity, orbital 65
Electronegativity, Pauling, L. 64
Electronegativity, Pearson, R.G. 64
Electronegativity, Rochow, E.G. 64
Electronegativity, Sanderson, R.T. 64
Electronic absorption spectra 76 379
Electronic absorption spectra and acid-base behavior 178
Electronic absorption spectra and electronic structure 341
Electronic absorption spectra, coordination compounds 388 390 392 394 398—402 406—408
Electronic absorption spectra, free-ion terms 391
Electronic absorption spectra, Laporte selection rule 390 406
Electronic absorption spectra, vibronic coupling 390
Electronic structure of coordination complexes, angular overlap method 342 346 361—368 371
Electronic structure of coordination complexes, crystal field theory 342 343 345 346
Electronic structure of coordination complexes, ligand field theory 342 345—347 349 351—357 360 361
Electronic structure of coordination complexes, valence bond theory 342 343 346
Electronic transitions in 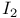 adducts 179 adducts 179
Electronically equivalent species 556—558
Electrophile-nucleophile acid-base definition 166
Electrophilic substitution, acetylace-tone complexes 449
Elements, geochemical classification 10
Ellis, A.B. 237
Emerald, color 379
Emission spectra and the Bohr atom 17
Enantiomers 310
Encapsulated metals in fullerenes 492 495
Energy bands in solids 138 223—227
Energy level splitting and overlap 40
Energy levels and spectra 19
Energy levels and spectra, homonuclear diatomic molecules 126
Energy levels and spectra, transition elements 42
Energy match and molecular orbital formation 122 138 145
Entering groups, effect on rate 435—437
Entering groups, rate constants and LFER parameters 436
Enterobactin 605 606
Enthalpy change by Hess’s Law 193
Enthalpy change from temperature dependence of equilibrium constant 193
Enthalpy of acid-base reaction 192
Enthalpy of adduct formation 192
Enthalpy of formation, ionic compounds 220
Enthalpy of hydration of bivalent ions 351 352
Enthalpy of hydration of bivalent ions, LFSE 351 352
Enthalpy of hydration of bivalent ions, simulated 375
Enthalpy of reaction, complex formation 338
Entropy change by temperature dependence of equilibrium constant 193
Entropy change from Hess’s Law 193
Entropy of acid-base reaction 192
Environmental chemistry 624—635
Enzymes, metal-containing 595
Epicurus 15
Equilibrium constant, temperature dependence 193
Ethane 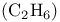 , symmetry 79 82 86—88 , symmetry 79 82 86—88
Ethylene 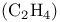 , as ligand 479 , as ligand 479
Exchange energy 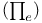 35—37 347 349 351 35—37 347 349 351
Expanded shells 53
Expanded shells, molecular orbitals 161
Expanding Universe 6
F (front) strain 199
Face centered cubic (fcc) 209 210 212
Fajans, K., rules of covalency 181
Farach, H.A. 237
Fast kinetics 415
Fast reactions (labile complexes), electronic structures 415
Fast reactions (labile complexes), measurement 422
Fe-protoporphyrin IX 597
Fergusson, J.E. 11 14 635
Fermi level 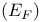 in semiconductors 225—227 in semiconductors 225—227
Ferredoxin 595 601 602
Ferrichromes 605
Ferrioxamines 605
Ferritin 604
Ferrocene, 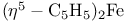 , bonding 486 489 , bonding 486 489
Ferrocene, 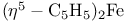 , conformation 457 458 , conformation 457 458
| Ferrocene, 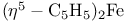 , molecular orbitals 487 489 , molecular orbitals 487 489
Ferrocene, 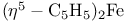 , reactions 490 , reactions 490
Ferrocene, 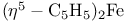 , synthesis 457 , synthesis 457
Figgis, B.N. 343
Finke, R.G 527 551
Finlayson-Pitts, B.J. 636
First row anomaly 245
Fischer, E.O. 498
Fischer-Tropsch process 550
Fischer-type carbene complexes 498
Fission bomb 12
Five-coordinate molecules 58
Fluorine, bonding 287
Fluorine, isolation 285
Fluorite 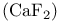 structure 216 structure 216
Fluoroantimonic acid 203
Fluorosulfonic acid 203
Fluxional behavior of complexes 328
Formal charge 53—55 691—695
Formal charge and expanded shells 55
Formaldehyde, photochemical smog 631
Formate dehydrogenase 595
Formation constants of complexes 337 338
Four-coordinate and six-coordinate preferences 373
Four-coordinate compounds 3
Framework molecular orbitals 572
Free ion terms 384—387 391—394
Free ion terms, 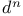 configurations 389 391 configurations 389 391
Friedel — Crafts alkylation, 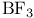 , catalyst 260 , catalyst 260
Friedel — Crafts catalysts 204
Frontier orbitals 137 558
Frontier orbitals and acid-base reactions 171—174
Frontier orbitals and Lewis acid-base definition 174
Frost diagrams, chlorine 288
Frost diagrams, hydrogen 246
Frost diagrams, nitrogen 278
Frost diagrams, oxygen 246
Fullerene-ferrocene hybrids 495
Fullerenes 4
Fullerenes as ligands 492 493
Fullerenes with encapsulated metals 492 493 495
Fullerenes, complexes 492—494
Fullerenes, intercalation compounds 492
Fullerenes, structures 264 265
Fullerenes, synthesis 265
Fulminate 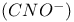 55 55
Fuming sulfuric acid (oleum) 203
Fusion bomb 12
GaAs, as LED 227
Gallium 260
Gamma rays 5 7
Gay-Lussac, J.L. 16
Geis, I. 598
Genesis of the elements 5
Geometric isomers 310
Geometries of inorganic compounds 3
Gerade, orbital symmetry 124
Gerloch, M. 47
Germanes, structure 271
Germanium 262
Gillard, R.D. 450 636
Gillespie, R.J. and ligand close packing 66 67
Gillespie, R.J. and VSEPR 57
Gimarc, B.M 162
Glutamate mutase 595
Gold complexes, in arthritis treatment 622
Gouy method for magnetic susceptibility 339
Grain boundaries 231
graphite 263 264
Gray, H.B. 635
Greenhouse effect 634
Greenhouse effect, 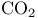 268 635 268 635
Greenhouse effect, methane 635
Greenhouse effect, SF5CF3 634
Greenwood, N.N. 14 237
Griffith, J.S. 304 343
Griffith, J.S., ligand field theory 12
Grignard reagent 255 457
Grignard, V. 457
Group 1 (IA) elements (alkali metals) 249—252
Group 13 (IIIA) elements 256—261
Group 14 (IVA) elements 261—271
Group 15 (VA) elements 272—279
Group 16 (VIA) elements 279—285
Group 17 (VIIA) elements (halogens) 285—290
Group 18 (VIIIA) elements (noble gases) 291—295
Group 2 (IIA) elements (alkaline earths) 253—255
Group orbitals 140
Group orbitals,  141—143 141—143
Group orbitals,  154—156 154—156
Group orbitals,  143—147 143—147
Group orbitals,  485 486 485 486
Group orbitals, 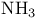 152 153 152 153
Group orbitals, definition 140
Group orbitals, use of 140 141 143 144 146 148—151 153 155 157
Group theory 82—102
Group theory, approach to bonding 139 140
Group theory, molecular orbitals 140—157
Group, mathematical 82—92
Group, mathematical, characters 96—102
Group, mathematical, matrices 92—97
Group, mathematical, properties 93
Groups, low and high symmetry 84
gyromagnetic ratio 341
Haber — Bosch process, ammonia synthesis 13 274
Haber, E., 1918 Nobel Prize 274
Half-life 6
Half-sandwich compounds 491
Halogens (Group 17) 17
Halogens (Group 17), energy levels 188
Halogens (Group 17), properties 286
Hamiltonian operator 21
Hammett acidity function 203
Handedness of chelate rings 315—317
Handedness of EDTA complex 316
Handedness of ligand ring conformation 318 319
Handedness of propellers and helices 315
Handedness, determining for chiral complexes 317
Hapticity 458 459
Hapto, organometallic nomenclature 458 459
Hard and soft acids and bases, theory (HSAB) 179—192
Hardness of acid or base 188
Hardness parameters 189
Hardness, absolute (n) 187—189 191
Hargittai, I. 110
Hargittai, M. 110
Hawking, S.W. 14
Heavy metal salts, carbonyl complex parallels 557
Heck, R.E 527
Hegedus.L.S. 551
Heisenberg, W., quantum mechanics 11 21
Heisenberg, W., uncertainty principle 19 21
Helium, burning 7
Helium, isolation 291
Helium, isotopes 6
Helium, properties 291
Heme group 597
Heme group, binding in hemoglobin 597 598
Heme group, color 379
Hemeryfhrin 595
Hemoglobin 5 595 597—599
Hemoglobin, 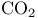 concentration 598 concentration 598
Hemoglobin, 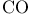 binding 598 599 binding 598 599
Hemoglobin, Bohr effect 598
Hemoglobin, color 379
Hemoglobin, model compounds 599
Hemoglobin, oxygen binding curve 598
Hemoglobin, pH effect 598
Hemoglobin, substitutes 599
Hess’s Law 193
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 as ligand
as ligand 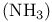
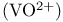 , acidity
, acidity 
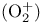
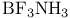
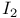 adducts
adducts 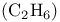 , symmetry
, symmetry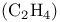 , as ligand
, as ligand 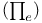
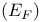 in semiconductors
in semiconductors 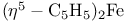 , bonding
, bonding 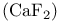 structure
structure 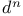 configurations
configurations 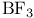 , catalyst
, catalyst 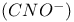
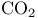
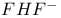

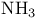
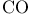 binding
binding