|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Miessler G., Tarr D.A. — Inorganic Chemistry |
|
|
 |
| Предметный указатель |
LUMO (lowest unoccupied molecular orbital) 127 137
LUMO (lowest unoccupied molecular orbital) and electron affinity 187
LUMO (lowest unoccupied molecular orbital), 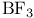 155 170 155 170
LUMO (lowest unoccupied molecular orbital), 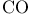 138 138
Lux — Flood acid-base definition 166
Lyman series, hydrogen spectrum 20
Madelung constant 220 221
Magic Acid 203
Magnesium properties and compounds 255
Magnetic levitation 229
Magnetic moment 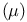 125 340 125 340
Magnetic moment 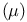 , spin only 340 341 , spin only 340 341
Magnetic quantum number  26 26
Magnetic susceptibilityof complexes 339 341
Magnetic susceptibilityof complexes, diamagnetic compounds 339
Magnetic susceptibilityof complexes, paramagnetic compounds 339
Magnitudes of  , ,  and and  368—370 368—370
Main group compounds, carbonyl complex parallels 556
Malachite, copper ore 11
Manhattan project 12
Mantle of Earth 10
Mathematical group 96
Matrices 92—97
Matrices, block diagonalized 96 99
Matrices, multiplication 93
Matrices, representation of a group 96
Maximum multiplicity, Hund’s rule 386 387
McCleverty, J.A. 450 636
McWeeny, R. 161
Mechanisms of substitution reactions 415
Mechanisms of substitution reactions, associative 419 425
Mechanisms of substitution reactions, conjugate base 426 427
Mechanisms of substitution reactions, dissociative 417
Mechanisms of substitution reactions, interchange 417 418
Medicinal compounds, inorganic 618 620
Mefhoxycarbene complex 499 500
Megatubes, carbon, structure 266
Meissner effect 228 229
Melting points, and adduct formation 192
Mendeleev, D.I., periodic table 11 12 16
Mercury, Arctic studies 626
Mercury, environmental 624 626
Mercury, lake studies 626
Metal alkyls 497
Metal clusters, carbon-centered 4
Metal hydroxides, solubility and acid-base strength 198
Metal to ligand   bonding 354 355 bonding 354 355
Metal to ligand charge transfer (MLCT) 408
Metal-carbon bonds 1 3
Metal-containing enzymes 5
Metal-metal bonds 1 566—570 572
Metal-metal bonds, multiple 567—570 572
Metallaboranes 579—581
Metallacarboranes 579—581
Metallacrowns 251
Metallacycles 497
Metallacyclobutadiene 547
Metallacyclobutane 547 549
Metallacyclobutane, synthesis 546
Metallaporphyrin 597
Metallocenes 485 489 490
Metalloids 242
Metals, properties 213
Metathesis 547
Metathesis, catalysts 546
Metathesis, Grubbs catalysts 545
Metathesis, olefin 544 545
Metathesis, ring-closing 545
Metathesis, Schrock catalysts 545
Methane, and greenhouse effect 634
Methionine synthetase 595
Methyl amine reactions 200
Methylation, by mefhylcobalamin 603
Methylcobalamin 603
Methylcobalamin and mefhylmercury synthesis 626
Meyer, L, periodic table 11 16
MICA 236
Microscopic reversibility, principle of 530
Microstate table 383
Microstates and quantum numbers 382—385 387
Miiller, K.A. 230
Millikan, R.A., electronic charge 17
Minamata Bay tragedy 625
Mine tailings 628
Minerals, types 10
Mirror planes 78 81 82 87 88
Mirror planes, dihedral 87 88
Mirror planes, horizontal 87
Mirror planes, reflection operation 101
Mirror planes, vertical 87 88
Mixing of orbitals 124 125 137
Mn(III), Jahn — Teller effect 372
mno rule 586
Molar absorptivity 381
Molecular dipoles 67 68
Molecular motions, character of 104
Molecular orbital theory 116
Molecular orbitals 76 117 122 160
Molecular orbitals and acid-base adducts 170—174
Molecular orbitals and band structure in solids 223—228
Molecular orbitals and photoelectrom spectrum of 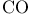 136 138 136 138
Molecular orbitals and photoelectron spectrum of 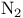 131 131
Molecular orbitals and photoelectron spectrum of 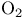 131 131
Molecular orbitals, 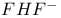 139 143 139 143
Molecular orbitals, 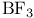 156 156
Molecular orbitals, 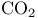 143 145—147 143 145—147
Molecular orbitals, 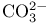 156 156
Molecular orbitals, 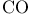 135—137 468 135—137 468
Molecular orbitals,  464 464
Molecular orbitals, 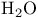 148 151 148 151
Molecular orbitals,  139 139
Molecular orbitals,  127 127
Molecular orbitals, 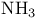 153 154 153 154
Molecular orbitals,  360 361 360 361
Molecular orbitals,  156 156
Molecular orbitals,  156 156
Molecular orbitals, azide ion,  147 147
Molecular orbitals, calculations for mechanistic studies 595
Molecular orbitals, cyanide ion 353
Molecular orbitals, energy match for formation 122 145
Molecular orbitals, framework, boranes 572
Molecular orbitals, from d orbitals 120 121
Molecular orbitals, from p orbitals 119
Molecular orbitals, homonuclear diatomic molecules 127—130
Molecular orbitals, hydrogen bonding in 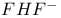 175 175
Molecular orbitals, isolobal fragments 559 563
Molecular orbitals, linear triatomic species 146
Molecular orbitals, octahedral complexes 345—347
Molecular orbitals, skeletal, boranes 572
Molecular orbitals, solid state 207
Molecular orbitals, square planar complex 356 357 466
Molecular orbitals, tetrahedral complexes 360 361
Molecular rearrangement processes 509
Molecular shapes and electronic structure 342
Molecular sieves 236
Molecular vibration, infrared active and inactive 104 106 108 109
Molecular wave function 116
Molina, M.J. 633
Mond process 473
Mond, L. 457
Monsanto Acetic Acid Process 538 539
Montmorillonite 236
Moore, E. 237
Moore, J.W. 450
Moseley, H.G.J. 100
Mulliken, R.S., electronegativity 64 187
Mulliken, R.S., electronegativity and acid-base theory 187
Multiple bonds and VSEPR 62 63
Multiple bonds in Be and B compounds 56 57
Multiple reflections 100
| Multiplicity 35
Mutagenic agents, requirements for 620
Myoglobin 597—599
Myoglobin, oxygen binding curve 598
n, principal quantum number 18 26 27 29
N-type semiconductor 225
Nano “onions”, fullerene 266
Nanotubes, structure 266
Natta, G., polymerization catalyst 12
Natural bond orbital method 161
Natural resonance theory 161
Neutrino 6
Neutron 6
Newton, D.E. 636
Ni(II) complexes 327
Nickelocene 489 490
Nido borane definition 574
Nitrate  structure and dipole moment 68 structure and dipole moment 68
Nitrate reductase 595 612
Nitric acid, properties 276
Nitric oxide synthase oxygenase 616 617
Nitric oxide,  276 276
Nitric oxide,  in biochemistry 616 618 in biochemistry 616 618
Nitric oxide,  , biosynthesis 616 , biosynthesis 616
Nitric oxide,  , vasodilator 616 , vasodilator 616
Nitride clusters 584
Nitrification 612
Nitrite reductase 595 612
Nitrite reductase, mechanism 613—615
Nitrite reductase, structure 615
Nitrogen 272—274
Nitrogen fixation 611
Nitrogen hydrides 274 275
Nitrogen isotopes 7
Nitrogen oxides 276
Nitrogen oxides and acid rain 630
Nitrogen oxides and ozone depletion 281
Nitrogen oxides and photochemical smog 630
Nitrogen oxides, reactions 276
Nitrogen-oxygen compounds 277
Nitrogenase enzymes 14 274 595 611
Nitrogenase enzymes, reactions 612
Nitrogenase enzymes, structure 611
Nitroglycerine, source of  617 617
Nitrous oxide,  276 276
NMR Spectra, of complexes 507 508
Noble gases (Group 18) 17
Noble gases (Group 18), chemistry 240 292
Noble gases (Group 18), compounds and ions 293
Noble gases (Group 18), electronegativity 65
Noble gases (Group 18), properties 292
nodes 29 30 32—34
Nodes in  systems 480 systems 480
Nodes, angular 32—34
Nodes, particle in a box 32
Nodes, planes,  486 486
Nodes, spherical (radial) 32 33
Nomenclature, coordination compounds 304 305 307 308
Nomenclature, organometallic 458 459
Nonaqueous solvents and acid-base strength 201 202
Nonbonding orbitals 118 120
Nonbonding orbitals, 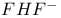 143 143
Nonbonding orbitals, octahedral complexes 346
Nonbonding orbitals, square planar complexes 356
Nonbonding pairs 51
Noncrossing rule 133
Nonhydrogenated oxygen atoms and acid strength 196 197
Nonsuperimposability 102
Normalization, wave function 22 23
Normalizing factor, N 117
Norton, J.R. 551
Nova 7
NS (thionitrosyl) 477
NS (thionitrosyl), complexes 476
Nuclear charge and atomic number 17
Nuclear magnetic resonance, and acid-base reactions 192
Nuclear reactions 6—8
Nuclear stability 7 8
Nuclear waste disposal 629
Nucleophilic discrimination factor 437
Nucleophilic displacement 526 527
Nucleophilic reactivity constant 437
Nyholm, R.S. 343
Nyholm, R.S., and VSEPR 57
OA (oxidative addition) 521 524 525 534 537 539
OA (oxidative addition), square planar 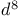 complexes 524 525 complexes 524 525
Ochiai, E.I. 600
Octahedral complexes 329
Octahedral complexes, molecular orbitals 345—347
Octahedral complexes, octahedral geometry 2
Octahedral complexes, VSEPR 58 61—63
Octahedral holes in crystal lattice 210
Octahedron, tetragonal distortion 330
Octet rule 52 53 55 56
Octet rule, Be and B compounds 56
Oil absorbent 236
Operator, Hamiltonian 21
Optical activity 76 102
Optical isomers 302 310
Optical rotatory dispersion (ORD) and chiral molecules 319 322 323
Orbital angular momentum quantum number (L) 384—387
Orbital angular momentum, total 382
Orbital interactions in octahedral complexes 345
Orbital mixing 124 125
Orbital potential energies 134 135
Orbital splitting  and electron spin 346 and electron spin 346
Orbital splitting  for aqueous ions 349 for aqueous ions 349
Orbitals 21 22 24—30 32 33
Orbitals, representations 101
Orbitals, shapes 26—30 32—34
Orbitals, used in bonding 76
Orbits, electron 21
Order of a group 98 100
Ore deposits 10
Organometallic catalysts 534
Organometallic chemistry 1 454
Organometallic compounds 3 454
Organometallic compounds, 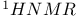 508 509 508 509
Organometallic compounds,  506 508 506 508
Organometallic compounds, catalysts 5 12
Organometallic compounds, characterization 509 511
Organometallic compounds, defined 299
Organometallic compounds, IR spectra 503 504 506
Organometallic compounds, main group parallels 556
Organometallic compounds, reactions 520
Organometallic nomenclature 458 459
Orgel, L.E., ligand field theory 12 304 343
Origin of the universe 5
Orthogonality of representations 98 100
Osmium tetroxide adducts 492
Ostwald, W., ions in aqueous solution 166
Outer orbital complexes 343
Overlap of atomic orbitals 117
Oxidation states and reaction rates 422
Oxidation states in organometallic compounds 521 524
Oxidation states, conditions for high and low 445
Oxidation-reduction reactions 245
Oxidation-reduction reactions of coordination compounds 440—442 444 445
Oxidation-reduction reactions, Frost diagram 246
Oxidation-reduction reactions, half reactions 245
Oxidation-reduction reactions, inner sphere mechanism 440—442 444 445
Oxidation-reduction reactions, Latimer diagram 245 246
Oxidation-reduction reactions, outer sphere mechanism 440—442 444 445
Oxidative addition (OA) 521 524 525 534 537 539
Oxidative addition (OA), square planar 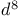 complexes 524 525 complexes 524 525
Oxide minerals 10
Oxo process 535 537
Oxonium ion 167
Oxyacids, strength of 196
Oxygen 280
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



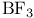
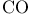
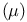

 ,
,  and
and 

 bonding
bonding 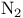
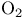
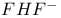
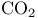
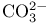

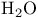


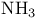




 structure and dipole moment
structure and dipole moment 


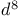 complexes
complexes  and electron spin
and electron spin