|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Miessler G., Tarr D.A. — Inorganic Chemistry |
|
|
 |
| Предметный указатель |
Oxygen, isolation 280
Oxygen, isotopes 7
Oxygen, properties 280
Oxygen, storage agents, hemoglobin and myoglobin 597—599
Oxygen-bonded carbonyls (isocarbonyls) 474
Oxyhemoglobin, electronic structure 599
Oyama, S.T. 551
Ozone  as oxidizing agent 281 as oxidizing agent 281
Ozone  , properties 280 281 , properties 280 281
Ozone layer 632—634
Ozone layer, chlorofluorocarbons 632
Ozone layer, depletion by chlorine, compounds and nitrogen oxides 281 632—634
Ozone layer, formation 632
Ozone layer, hole, Antarctic 632—634
Ozone layer, hole, Arctic 634
Ozone layer, protective action 281 632
Ozonide ion 281
p-dichlorobenzene, symmetry 82
p-n junction 226
P-type semiconductor 225
Pairing energy for aqueous ions 349
Pairing energy, electron 36
Paramagnetic compounds 125
Paramagnetic compounds, magnetic susceptibility 339
Paramagnetism of 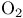 128 280 128 280
Particle in a box 23—25
Particle in a box, probability densities 25
Particle in a box, wave equation 23—25
Partington, J, R. 47
Paschen series, hydrogen spectrum 20
Pauli exclusion principle 35
Pauling, L. 304 343
Pauling, L., electronegativity 64
Pauling, L., strength of oxyacids 196 197
Pauson, P.L. 457
Pd(II) complexes 327
Pearson, R.G. 187 435 450
Pearson, R.G., electronegativity 64
Pearson, R.G., hard and soft acids and bases 183 187
Pentagonal bipyramidal geometry 331
Pentagonal bipyramidal geometry, VSEPR 58 60
Perchloric acid 203
Periodic properties of atoms 43 44 46 47
Periodic table 16 17
Periodic table, families, groups, periods 17
Permanganate 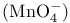 , acidity 198 , acidity 198
Peroxidases 595 600
Peroxide ion 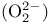 128 281 128 281
Peroxyacetyl nitrate and photochemical smog 632
peroxynitrite  278 278
Phosphine  , bond angle 66 , bond angle 66
Phosphine  , properties 275 , properties 275
Phosphine dissociation 522
Phosphine ligands,  -acceptor ability 506 -acceptor ability 506
Phosphine ligands,  -donor ability 506 -donor ability 506
Phosphohydrases 595
Phosphoric acid  synthesis 279 synthesis 279
Phosphorus 272 273
Phosphotransferases 595
Photochemical smog 627
Photochemical smog, formaldehyde 631
Photochemical smog, hydroxyl radical 630
Photochemical smog, nitrogen oxides 630
Photochemical smog, ozone 631
Photoelectron spectra and molecular orbitals of 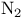 131 131
photons 6
Photosynthesis 600
Photovoltaic effect 226 227
Phthalocyanine synthesis 448
Pitts, J.N., Jr. 636
Planar trigonal geometry, VSEPR 58 60 62 63
Planck’s constant 18
Plane-polarized light 103
Planets, formation 8
Plastocyanin 595
Point groups 82—92
Point groups, assignment 82—92
Point groups, diagram 83
Point groups, examples 82—92
Polar bonds 67 134 138
Polarizability, and acid-base properties 183
Polonium 280
Polyatomic halide ions 288
Polyiodide ions 288
Polymerization of ethylene 12
Polymerization, metallacyclobutane intermediate 547 549
Polymerization, metallacyclobutane of norbornene using carbene catalyst 547
Polymers, 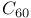 266 266
Poole, C.P., Jr. 237
Porphine 596 597
Porphyrins 596
Porphyrins, iron 597—600
Porphyrins, similar ring compounds 600 603 604
Portland cement 255
Positron 6 7
Potential energy, in quantum mechanics 21 22
Powell, H.M., VSEPR 57
Primitive cubic crystal structure 209
Principal axis 78 86
Principal quantum number (n) 18 26 27 29
probability 21
Probability, densities, particle in a box 25
Proper rotation axis 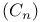 77 77
Properties and representations of groups 92
Properties of metals 213
Properties of solvents 169
Proteins,  -helix 71 72 -helix 71 72
Proteins, metal-containing 595
Proteins, pleated sheet 71 72
Protic solvents 169
Proton 5 6
Proton affinity 194 200
Protonated species, formation of 192
Prussian blue ![$\mathrm{(KFe[Fe(CN)_6])}$](/math_tex/2a985a88dc1153d8aec11e8e6ddb2de982.gif) 299 379 299 379
Pseudohalogens 290
Pseudorotation 434
Pt(II) complexes 327
Pyridine, as solvent 203
Pyridines, basicity of substituted 199
Pyrocatechase 595
Pyrophyllite 235
Pyruvate phosphokinase 595
Quadruple bonds between metal atoms 1 568—572
qualitative analysis 12 185
Quantum numbers and atomic wave functions 25—29 32—34
Quantum numbers, angular momentum (l) 26—28
Quantum numbers, magnetic  26—28 26—28
Quantum numbers, microstates 382—385 387
Quantum numbers, multielectron atoms 382—385 387
Quantum numbers, particle in a box 24 25
Quantum numbers, principal (n) 18 24 26—28
Quantum numbers, properties 26
Quantum numbers, spin  26—28 26—28
Quantum numbers, total angular momentum (J) 384—387
Quantum numbers, total orbital angular momentum (L) 340 384—387
Quantum numbers, total spin angular momentum (S) 340 384—387
Quantum theory of the atom 18
Quartz structure 233
Radial function, R 27—29
Radial nodes 32
Radial probability functions 29 32
Radial wave function 28—30 32
Radioactive waste 629
Radioactivity 8 11
Radius ratio 215 218 219
Radius ratio and coordination number 219
Radius, covalent 44 45
Radius, crystal 44—47
Radius, ionic 44—47 668—671
Radon 291 292
| Rate constants for ![$\mathrm{[Ru(II)(EDTA)(H_2O)]^{2-}}$](/math_tex/561ce37b1fa6540968b484a27008d0c082.gif) , substitution 426 , substitution 426
Rate constants for leaving groups 437
Rate constants for reactions of ![$\mathrm{[Co(en)_2(H_2O)X]$](/math_tex/1b38f44559a4b406cd8ca3ba54894cff82.gif) {n+}}$ 432 {n+}}$ 432
Rate constants for [Ru(III)(EDTA)( )r, substitution 426 )r, substitution 426
Ratio of 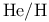 in universe 6 in universe 6
RE (Reductive elimination) 521 524 525 534 539
Redox potentials 245
Redox potentials, changed by coordination 595
Redox potentials, chlorine 288
Redox potentials, hydrogen 245
Redox potentials, nitrogen 278
Redox potentials, oxygen 246
Reduced mass, 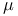 18 469 18 469
Reducible representations 96 97 104—107
Reducible representations, characters of 96 97
Reducible representations, hybrid orbitals 159 160
Reducible representations, reducing to irreducible representations 105—107
Reductive carbonylation 474
Reductive elimination (RE) 521 524 525 534 539
Reflection operation ( ) 78 79 81—84 87 88 90—95 ) 78 79 81—84 87 88 90—95
Reflection operation ( ), dihedral 79 87 88 101 ), dihedral 79 87 88 101
Reflection operation ( ), horizontal 79 87 88 101 ), horizontal 79 87 88 101
Reflection operation ( ), vertical 79 87 88 101 ), vertical 79 87 88 101
Replacement, ligand 521
Representations 96
Representations of octahedral ligand orbitals 345 346 353
Representations of point groups 94—97
Representations of square planar complexes 357
Representations of tetrahedral complexes 360
Representations, irreducible 96—97
Representations, notation 101 102
Representations, reducible 96 97
Representations, reducing 105 106
Representations, rotational 105 106
Representations, totally symmetric 98
Representations, translational 105 106
Representations, vibrational 105 106 108—110
Resonance 52
Resonance, 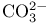 52 52
Resonance, thiocyanate 54
Reversibility, principle of microscopic 530
Rich, R.L. 41 42
Rochow, E.G., electronegativity 64
Rotation axes 77 78 81 83 86 87
Rotation operation (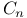 ) 77 ) 77
Rotation, effect on coordinates of a point 100
Rotation-reflection axis (improper axis, 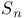 ) 79 81 ) 79 81
Rotational representations 108
Rotational symmetry ( ) 101 104 106 ) 101 104 106
Rowland.F.S. 633
Ruby, color 379
Russell — Saunders coupling 382
Rutherford, E., nuclear atom 17
Rutile 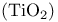 crystal structure 217 crystal structure 217
Rydberg constant 18
S, quantum number 340
SALC, symmetry-adapted linear combinations 140
Sanderson, R T., electronegativity 64
Sandwich compounds 4 454 455 485
Sandwich compounds, examples 455
Sandwich compounds, multiple-decker 4
Sasol plants in South Africa 550
Schrieffer, J.R. 229
Schrock metathesis catalysts 545
Schrock-type carbene complexes 498
Schrodinger equation, three- dimensional 25
Schrodinger, E., quantum mechanics 11
Schrodinger, E., wave equation 21—24 26—29 32—36 38 40 41 43
Schwederski, B. 635
Seesaw geometry, VSEPR 61
Selection rules, spectra 390
Selenium 279 280 285
Selenocarbonyl (CSe) 475
Self-interstitials 232
Semiconductors, conductance 224
Semiconductors, defined 224
Semiconductors, doped 224
Semiconductors, energy bands, temperature dependence 225
Semiconductors, Fermi level 225—227
Semiconductors, intrinsic 224
semiconductors, N-type 225
semiconductors, P-type 225
Semiconductors, temperature dependence of conductance 224
Semimetals 242
Seven-coordinate molecules 58
Shannon, R.D 46
Shape memory metals 214
shielding 38 40 41 43
Shielding constant S 38 40 41
Siderochromes 605
Siderophiles 10
Sidgwick, N.V., and VSEPR 57
Silanes, comparison with carbon compounds 271
Silanes, structure 271
Silica gel 269
Silica, 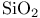 , structure 232 233 , structure 232 233
Silicates, minerals 10
Silicates, structures 232—237 269 270
Silicon carbide 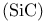 structure 271 structure 271
Silicon compounds 269
Silicon dioxide 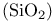 232 233 232 233
Silicon, isolation 262
Silver chloride solubility, thermodynamics 222
Silver halide solubility and acid-base properties 179
Sixteen-electron complexes, square planar 465—467
Skeletal bonding orbitals 572
Slater’s rules, shielding 38 40
Slow reactions (inert complexes), electronic structures 415
Smart, L. 237
Snowflake, symmetry 77 78
Sodium chloride structure 214 215
Sodium hydroxide 203
Soft acids and bases 180—186
Softness of acid or base 188
Solid-state laser 227 228
Solubility and HSAB 183 185 222
Solubility and ion size 222
Solubility of metal hydroxides 198 199
Solubility product constants 198
Solvation and acid-base strength 200 201
Solvent isomers 309
Solvent system, acid-base definition 166 168
Somorjai, G.A. 551
Spectra of 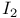 with different bases 180 with different bases 180
Spectral analysis of organometallic complexes 503
Spectrochemical series 367 368
Spherical coordinates 28
Spherical nodes 32 33
Spin angular momentum quantum number, S 384—387
Spin angular momentum, total 382
Spin magnetic moment 340
Spin quantum number, 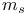 26 27 26 27
Spin states and ligand field strength 348
Spin, electron 27
Spin-allowed transitions 390
Spin-orbit coupling 387
Splitting of free ion terms in octahedral symmetry 392
Splitting, F, octahedral symmetry 403
Splitting, Jahn — Teller distortion 400
Square antiprismatic geometry 332 333
Square antiprismatic geometry, VSEPR 58—60
Square-planar complexes 3 327 328
Square-planar complexes, 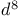 complexes, OA reactions 524 525 complexes, OA reactions 524 525
Square-planar complexes,  bonding 357 360 bonding 357 360
Square-planar complexes,  bonding 360 bonding 360
Square-planar complexes, electron counting 465 466
Square-planar complexes, electronic structure 342
Square-planar complexes, isomers 310
Square-planar complexes, molecular orbitals 356—360 466
Square-planar complexes, Pd(II) and Pt(II) complexes 327
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 as oxidizing agent
as oxidizing agent 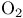
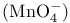 , acidity
, acidity 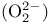

 , bond angle
, bond angle  -acceptor ability
-acceptor ability  -donor ability
-donor ability  synthesis
synthesis 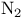
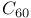
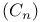
 -helix
-helix ![$\mathrm{(KFe[Fe(CN)_6])}$](/math_tex/2a985a88dc1153d8aec11e8e6ddb2de982.gif)


![$\mathrm{[Ru(II)(EDTA)(H_2O)]^{2-}}$](/math_tex/561ce37b1fa6540968b484a27008d0c082.gif) , substitution
, substitution ![$\mathrm{[Co(en)_2(H_2O)X]$](/math_tex/1b38f44559a4b406cd8ca3ba54894cff82.gif) {n+}}$
{n+}}$ 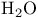 )r, substitution
)r, substitution 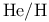 in universe
in universe 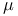
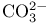
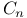 )
) 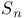 )
) 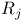 )
) 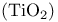 crystal structure
crystal structure 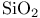 , structure
, structure 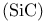 structure
structure 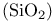
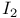 with different bases
with different bases 
 complexes, OA reactions
complexes, OA reactions