|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Miessler G., Tarr D.A. — Inorganic Chemistry |
|
|
 |
| Предметный указатель |
 -donor interactions, angular overlap 362 -donor interactions, angular overlap 362
 -donor ligands, octahedral complexes 362—364 366 -donor ligands, octahedral complexes 362—364 366
 -donor orbitals 463 -donor orbitals 463
 , mirror plane 101 , mirror plane 101
 , perpendicular mirror plane 101 , perpendicular mirror plane 101
 , mirror plane 101 , mirror plane 101
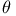 , angular function 28 , angular function 28
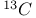 NMR of complexes 507 508 NMR of complexes 507 508
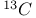 NMR spectrometry 262 NMR spectrometry 262
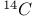 and radiocarbon dating 262 and radiocarbon dating 262
 of complexes 508 509 of complexes 508 509
(Dipicolinato)oxovanadate(V), insulin-like activity 622
1, 3, 5, 7—Tetrafluorocyclooctatetraene, symmetry 87 88
1, 3-butadiene, as ligand 480
1, 5-DibromonaphthaIene, symmetry 87
Abel, E.W. 551
Absolute electronegativity 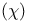 187—189 187—189
Absolute hardness 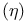 187—189 674 675 187—189 674 675
Absorbance 381
Absorption of light 380
Absorption spectra, ![$[\mathrm{Cr(H_2O)_6}]^{2+}$](/math_tex/9f0e96c3ec2faceb713e8d0666080d8182.gif) 401 401
Absorption spectra, ![$[\mathrm{Cr(H_2O)_6}]^{3+}$](/math_tex/928d61bdbf8276bec7e7974c9efd6f6982.gif) 402 402
Absorption spectra, ![$[\mathrm{Cu(H_2O)_6}]^{2+}$](/math_tex/3b1b18fd8a7467f8a877aa248bddf8fe82.gif) 398^101 398^101
Absorption spectra, ![$[\mathrm{Fe(H_2O)_6}]^{2+}$](/math_tex/51fac492e4dc2c01f3527021d6e9e98282.gif) 401 401
Absorption spectra, ![$[\mathrm{M(H_2O)_6}]^{n+}$](/math_tex/6235d9368dd208e133b749ebbe856d1982.gif) 397 398 397 398
Absorption spectra, ![$[\mathrm{Mn(H_2O)_6}]^{2+}$](/math_tex/61769bcf6edf745851f04494226b010082.gif) 397 405 397 405
Absorption spectra, ![$[\mathrm{Ni(H_2O)_6}]^{2+}$](/math_tex/afda54340afa07b4657f379da5f55a4582.gif) 402 402
Absorption spectra, ![$[\mathrm{Ti(H_2O)_6}]^{3+}$](/math_tex/581caaae1d9102cd23fe782bbde9c50782.gif) 398 401 398 401
Absorption spectra, ![$[\mathrm{V(H_2O)_6}]^{3+}$](/math_tex/8fa7558d560b7f86d46eb4145c23872482.gif) 394 404 394 404
Absorption spectra, Bohr atom 18
Absorption spectra, coordination compounds 388
Absorption spectra, Tetrahedral complexes 390 406
Abstraction reactions 534
Abundances of the elements, cosmic 8
Acetaldehyde, synthesis from ethylene 541
Acetic acid dissociation, thermodynamics 193
Acetic acid process, Monsanto 538 539
Acetylene  , symmetry 91 , symmetry 91
Acetylide, structure 268
Acid and base strength 203
Acid rain 630
Acid-base activity, changed by coordination 595
Acid-base concepts as organizing concepts 165
Acid-base concepts, Arrhenius 166—168
Acid-base concepts, Bronsted — Lowry 166—168
Acid-base concepts, frontier orbitals 171—174
Acid-base concepts, Ingold — Robinson 166
Acid-base concepts, Lavoisier 165 166
Acid-base concepts, Lewis 166 170 171
Acid-base concepts, Liebig 165 166
Acid-base concepts, Lux — Hood 166
Acid-base concepts, solvent system 166 168—170
Acid-base concepts, summary 166
Acid-base concepts, Usanovich 166
Acid-base definitions 166
Acid-base parameters, quantitative 187—194
Acid-base properties, binary hydrogen compounds 194
Acid-base properties, binary hydrogen compounds and frontier orbitals 171—174
Acid-base properties, binary hydrogen compounds, quantitative measures of 187—194
Acid-base strength 192—202
Acid-base strength of cations 197—199
Acid-base strength of cations and nonaqueous solvents 201 202
Acid-base strength of cations and solvation 200 201
Acid-base strength of cations and solvents 200
Acid-base strength of cations, inductive effects 196
Acid-base strength of cations, inherent 184 186 188 191 199
Acid-base strength of cations, oxyacids 196 197
Acid-base strength of cations, proton affinity 194
Acid-base strength of cations, quantitative measures 187—194
Acid-base strength of cations, steric effects 199 200
Acid-base strength of cations, thermodynamic measurement 193 194
Acid-base strength, binary hydrogen compounds 194 195
Acidity and electronegativity, trends 195
Acids and bases with parallel changes in E and C 191
Aconitase 595
Actinides 17
Activation energies and reaction enthalpies 421
Addition reactions 521 524 525
Addition to unsaturated species, halogens and carbonyl compounds 557
Adduct formation 192
Adduct formation, acid-base 170—176 178 179 181 182 186
Adenosine triphosphate (ATP) 600
Ahrland, S. 182
Alchemy 11
Alcohol dehydrogenase 595
Alizarin red dye 299
Alkali metals (Group 1) 17 249—253
Alkali metals (Group 1), anions 240 251
Alkali metals (Group 1), chemical properties 249 250
Alkali metals (Group 1), combustion products 250
Alkali metals (Group 1), cryptands 252
Alkali metals (Group 1), isolation 249
Alkali metals (Group 1), properties 249
Alkali metals (Group 1), solutions in liquid ammonia 250
Alkalides 251
Alkaline earth metals (Group 2) 17 253
Alkaline earth metals (Group 2), chemical properties 254
Alkaline earth metals (Group 2), sources 253
Alkaline earth metals (Group 2), uses 254
Alkenyl (vinyl) ligands 498
Alkyl complexes 497
Alkyl complexes, bonding 496
Alkyl complexes, synthesis 496 497
Alkyl groups, bridging 1 3
Alkyl groups, effect on base strength 196
Alkyl groups, terminal 1 3
Alkyl ligands 496 497
Alkyl migration mechanism 528—532
Alkyne metathesis 547
Alkynyl ligands 498
Allen, L.C., electronegativity 64
Allene  , symmetry 90 , symmetry 90
Allred, A.L., electronegativity 64
Allyl complexes 483 484
Aluminosilicates 10
Aluminosilicates, kaolinite 234
Aluminosilicates, montmorillonite 236
Aluminosilicates, pyrophyllite 234
Aluminosilicates, structure 234 236 237
Aluminosilicates, zeolites 236
Aluminum compounds, bridged 259
Aluminum, properties 260
Ambidentate isomerism 309
Amethyst, color 379
Amide ion  structure and shape 62 structure and shape 62
Amine oxidases 595
Amines, basicity of 196
Ammonia, basicity and frontier orbitals 172
Ammonia, boiling point 69
Ammonia, bond angle 59 60
Ammonia, dimer 70
Ammonia, electron repulsion in 60
Ammonia, group orbitals 151
Ammonia, Haber — Bosch process 13
Ammonia, hybrid orbitals 158
Ammonia, hydrogen bonding 70
Ammonia, molecular orbitals 152—154
Ammonia, symmetry operations 92
Ammonia, synthesis 13 274
Ammonia, synthesis by nitrogenase 611
Ammonia, uses 274
Ammonium ion  structure and shape 62 structure and shape 62
Ammonium nitrate, uses 276
Amphibole asbestos 236
Amphoteric 201
Anation 422
and molecular orbitals of 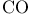 136 136
and molecular orbitals of 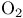 131 131
Anesthesia, theory of 71
Angels 32
| Angular functions,  26 29 26 29
Angular functions, 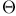 26 29 26 29
Angular functions, Y 33
Angular momentum quantum numbers, J 384—387
Angular momentum quantum numbers, l 26 27 29
Angular nodal surfaces 30 32 33
Angular overlap model 342
Angular overlap model,  acceptor interactions 364 365 367 acceptor interactions 364 365 367
Angular overlap model,  donor Interactions 366 367 donor Interactions 366 367
Angular overlap model,  donor interactions 362—365 donor interactions 362—365
Angular overlap model, 4- and 6-coordinate preferences 373 374
Angular overlap model, ligand field theory 362—368 371
Angular overlap model, magnitudes of  , ,  , and , and  368—371 368—371
Angular overlap model, other shapes 375
Angular overlap model, parameters 371
Angular overlap model, special cases 369
Angular overlap model, trigonal bipyramidal complexes 375 376
Antibonding molecular orbitals 117 118 120
Antibonding molecular orbitals, 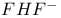 143 143
Antibonding molecular orbitals, octahedral complexes 346
Antifluorite structure 217
Antimony 272 274
Antineutrino 5 6
Antisymmetric Stretch of CO 504
Antitumor agent, cisplatin 618
Antitumor agent, cisplatin, mechanism 620
Apoferritin 604
Aprotic solvents 168 169
Aquation 422
Arachno borane definition 574
Aragonite structure 218
Argon 291
Aromatic rings as ligands 3
Arrhenius, S., acid-base definition 166
Arsenic, Bangladesh water supply 628
Arsenic, environmental 628
Arsenic, properties 272 274
Arsine  bond angle 66 bond angle 66
Arsines  276 276
Arthritis treatment, auranofin 622
Aryl ligands 498
Asbestos 236
Ascorbate oxidase 595
Associative interchange mechanism  415 415
Associative mechanism (A) 419
Associative mechanism (A), evidence for 435—437
Associative mechanism (A), Ru(III) compounds 425
Associative mechanism (A), substitution in octahedral complexes 425
Associative property, in a group 93
Astatine 285
Atmophiles 10
Atomic orbitals 27 33
Atomic orbitals, mathematics of 26 28 29
Atomic orbitals, order of filling 38
Atomic orbitals, positive and negative signs 117—122
Atomic orbitals, shapes 25 27—29 31—33
Atomic theory, historical development 15—19 21 22
Atomic wave functions 21—30 32 33
Atoms and molecules, concept 11
ATPase 595
Atwood, J.D. 450
Aufbau principle 34
Auranofin, antiarthritis treatment 622
Aureolin ![$\mathrm{(K_3[Co(NO_2)_6]\dot 6H_20)}$](/math_tex/d430331608550b5435d963a7173c03ed82.gif) 299 299
Avogadro, A. 16
Background radiation 6
Bacon, Roger 11
Bailar twist 434
Bailey, R.A. 635
Baldwin, J. 598
Ball and chain dimers, fullerene 266
Balmer series, hydrogen spectrum 17—20
Band gap in solids 223 225—227
Band structure in solids 223 225—227
Bardeen, J. 229
Base strength, inherent 184
Basolo, R 435 450
Bauxite (hydrated  10 10
Becquerel, H., radioactivity 11 17
Bednorz, J.G. 230
Beer — Lambert absorption law 380 381
Bennett, W.E. 314
Benzene and borazine comparison 261
Benzene, symmetry 90
Berg, J.M. 635
Bertini 1.
Beryllium 254
Beta elimination 533
Bethe, H.A., crystal field theory 12 304 344
Bidentate ligands 307
Big bang theory 5
Big bang theory, background radiation 6
Bimolecular mechanism 522
Binary carbonyl complexes 472 473
Binary hydrogen compounds, acidity and basicity 194 195
Bismuth 272 274
Bleach, chlorine and bromine 286
Block diagonalized matrix 96 97
Blomstrand, C.W., chain theory 300
Bockris, J.O’M. 635
Body-centered cubic 209—213 216 217
Bohr atom 17—19
Bohr magneton  340 340
Bohr radius  29 29
Bohr, N., atomic theory 11 18 19
Boiling point and adduct formation 171 192
Boiling point and hydrogen bonding 70
Bond angles and electronegativity 66
Bond angles and size of central atom 66
Bond angles, 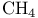 59 59
Bond angles, 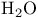 59—61 59—61
Bond angles, 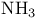 60 61 60 61
Bond angles, VSEPR 58—63 65—67
Bond dipoles 67—69
Bond lengths, and VSEPR 66 67
Bond order 123
Bond order and electron count, dimetal clusters 570
Bond polarity 67 68
Bonding in ionic crystals 231
Bonding interactions between metal d orbitals 569
Bonding molecular orbitals 118 120
Bonding of  to metal 493 to metal 493
Bonding orbitals,  143 143
Bonding orbitals, octahedral complexes 346
Bonding pair-bonding pair (bp-bp) repulsion, VSEPR 57 59—63
Bonding pair-lone pair (bp-lp) repulsion, VSEPR 60—63
Bonding, 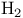 118 118
Boranes 259 572 574 577
Boranes, classification 574 577
Boraphosphabenzenes 261
Borazine and benzene, comparison 261
Born — Haber cycle 220
Boron nitride (BN) properties 261
Boron trifluoride-diethyl ether, adduct,  \mathrm{O(C_2H_5)_2}$ 171 \mathrm{O(C_2H_5)_2}$ 171
Boron trihalides  as Lewis acids 260 as Lewis acids 260
Boron, compounds 256
Boron, hydrides, see boranes 572
Boron, isotopes 259
Boron, properties 256
Boron, uses 259
Bravais lattices 208
Bridging ligands, alkyl groups 1 2
Bridging ligands, carbonyls (CO) 240 473
Bridging ligands, hydrogen atoms 1—3 240
Bridging modes of CO 470 471
Bromine 285
Bronsted — Lowry acid-base, definition 166—168
Bronsted, J.N 166 167
Brosset, C 567
Brown, H.C 199
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -donor interactions, angular overlap
-donor interactions, angular overlap  , mirror plane
, mirror plane  , perpendicular mirror plane
, perpendicular mirror plane  , mirror plane
, mirror plane 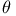 , angular function
, angular function 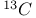 NMR of complexes
NMR of complexes 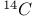 and radiocarbon dating
and radiocarbon dating  of complexes
of complexes 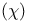
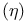
![$[\mathrm{Cr(H_2O)_6}]^{2+}$](/math_tex/9f0e96c3ec2faceb713e8d0666080d8182.gif)
![$[\mathrm{Cr(H_2O)_6}]^{3+}$](/math_tex/928d61bdbf8276bec7e7974c9efd6f6982.gif)
![$[\mathrm{Cu(H_2O)_6}]^{2+}$](/math_tex/3b1b18fd8a7467f8a877aa248bddf8fe82.gif)
![$[\mathrm{Fe(H_2O)_6}]^{2+}$](/math_tex/51fac492e4dc2c01f3527021d6e9e98282.gif)
![$[\mathrm{M(H_2O)_6}]^{n+}$](/math_tex/6235d9368dd208e133b749ebbe856d1982.gif)
![$[\mathrm{Mn(H_2O)_6}]^{2+}$](/math_tex/61769bcf6edf745851f04494226b010082.gif)
![$[\mathrm{Ni(H_2O)_6}]^{2+}$](/math_tex/afda54340afa07b4657f379da5f55a4582.gif)
![$[\mathrm{Ti(H_2O)_6}]^{3+}$](/math_tex/581caaae1d9102cd23fe782bbde9c50782.gif)
![$[\mathrm{V(H_2O)_6}]^{3+}$](/math_tex/8fa7558d560b7f86d46eb4145c23872482.gif)
 , symmetry
, symmetry  , symmetry
, symmetry  structure and shape
structure and shape  structure and shape
structure and shape 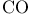
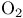

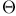
 acceptor interactions
acceptor interactions  ,
,  , and
, and 
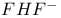
 bond angle
bond angle 
![$\mathrm{(K_3[Co(NO_2)_6]\dot 6H_20)}$](/math_tex/d430331608550b5435d963a7173c03ed82.gif)



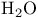
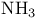
 to metal
to metal 
 \mathrm{O(C_2H_5)_2}$
\mathrm{O(C_2H_5)_2}$  as Lewis acids
as Lewis acids